Abstract
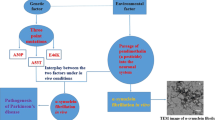
The formation of amyloid-like fibrils, which polymerize from various soluble proteins under physiological and acidic conditions, causes a wide range of protein-folding diseases, such as Alzheimer’s disease and Parkinson’s disease. Fibril assembly in in vitro solutions containing nitric oxide, a free radical that functions as an important signalling molecule involved in numerous physiological and pathological processes, has not been reported. Here, we investigated the protein assembly that occur in thyroglobulin under mildly acidic conditions in the presence of nitric oxide. Solution studies, size exclusion chromatography, dynamic light scattering and analytical ultracentrifugation, demonstrated the size changes of thyroglobulin oligomers after nitric oxide treatment. Following electron microscopic analysis visualized their structural changes and revealed that the molecules can morphologically form polymerized fibril assemblies with a length of 2–5 μm and width 10–100 nm. Taken together, these results provide suggestive evidence for the propensity of forming polymerized thyroglobulin fibrils implying their presence in thyroid cells, which may be related to the onset or progression of thyroid diseases.






Similar content being viewed by others
Abbreviations
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- Tg:
-
Thyroglobulin
- T3 :
-
Triiodothyronine
- T4 :
-
Thyroxine
- ChEL:
-
Cholinesterase-like
- SEC:
-
Size exclusion chromatography
- EM:
-
Electron microscopy
- DLS:
-
Dynamic light scattering
- AUC:
-
Analytical ultracentrifugation
- PBS:
-
Phosphate-buffered saline
References
Dobson CM (2001) The structural basis of protein folding and its links with human disease. Philos Trans R Soc Lond B Biol Sci 356:133–145
De Jong KL, Incledon B, Yip CM, DeFelippis MR (2006) Amyloid fibrils of glucagon characterized by high-resolution atomic force microscopy. Biophys J 91:1905–1914
Chiti F, Stefani M, Taddei N, Ramponi G, Dobson CM (2003) Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature 424:805–808
Lopez de la Paz M, Serrano L (2004) Sequence determinants of amyloid fibril formation. Proc Natl Acad Sci USA 101:87–92
Weijers M, Broersen K, Barneveld PA, Cohen Stuart MA, Hamer RJ, De Jongh HH, Visschers RW (2008) Net charge affects morphology and visual properties of ovalbumin aggregates. Biomacromolecules 9:3165–3172
Saito S, Ando Y, Nakamura M, Ueda M, Kim J, Ishima Y, Akaike T, Otagiri M (2005) Effect of nitric oxide in amyloid fibril formation on transthyretin-related amyloidosis. Biochemistry 44:11122–11129
Reynolds MR, Berry RW, Binder LI (2005) Site-specific nitration and oxidative dityrosine bridging of the tau protein by peroxynitrite: implications for Alzheimer’s disease. Biochemistry 44:1690–1700
Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM (2000) Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290:985–989
Horiguchi T, Uryu K, Giasson BI, Ischiropoulos H, LightFoot R, Bellmann C, Richter-Landsberg C, Lee VM, Trojanowski JQ (2003) Nitration of tau protein is linked to neurodegeneration in tauopathies. Am J Pathol 163:1021–1031
Fozzatti L, Velez ML, Lucero AM, Nicola JP, Mascanfroni ID, Maccio DR, Pellizas CG, Roth GA, Masini-Repiso AM (2007) Endogenous thyrocyte-produced nitric oxide inhibits iodide uptake and thyroid-specific gene expression in FRTL-5 thyroid cells. J Endocrinol 192:627–637
Akaike T, Maeda H (2000) Nitric oxide and virus infection. Immunology 101:300–308
Kikugawa K, Hiramoto K, Ohkawa T (2004) Effects of oxygen on the reactivity of nitrogen oxide species including peroxynitrite. Biol Pharm Bull 27:17–23
Bloth B, Bergquist R (1968) The ultrastructure of human thyroglobulin. J Exp Med 128:1129–1136
Dunn JT, Dunn AD (1999) The importance of thyroglobulin structure for thyroid hormone biosynthesis. Biochimie 81:505–509
Arvan P, Di Jeso B (2004) The Thyroid, 9th edn. Lippincott Williams & Wilkins, Philadelphia
Lamas L, Taurog A (1977) The importance of thyroglobulin structure in thyroid peroxidase-catalyzed conversion of diiodotyrosine to thyroxine. Endocrinology 100:1129–1136
van de Graaf SA, Ris-Stalpers C, Pauws E, Mendive FM, Targovnik HM, de Vijlder JJ (2001) Up to date with human thyroglobulin. J Endocrinol 170:307–321
Belkadi A, Jacques C, Savagner F, Malthiery Y (2012) Phylogenetic analysis of the human thyroglobulin regions. Thyroid Res 5:3
Lee J, Di Jeso B, Arvan P (2011) Maturation of thyroglobulin protein region I. J Biol Chem 286:33045–33052
Lee J, Wang X, Di Jeso B, Arvan P (2009) The cholinesterase-like domain, essential in thyroglobulin trafficking for thyroid hormone synthesis, is required for protein dimerization. J Biol Chem 284:12752–12761
Wang X, Lee J, Di Jeso B, Treglia AS, Comoletti D, Dubi N, Taylor P, Arvan P (2010) Cis and trans actions of the cholinesterase-like domain within the thyroglobulin dimer. J Biol Chem 285:17564–17573
Hishinuma A, Fukata S, Nishiyama S, Nishi Y, Oh-Ishi M, Murata Y, Ohyama Y, Matsuura N, Kasai K, Harada S, Kitanaka S, Takamatsu J, Kiwaki K, Ohye H, Uruno T, Tomoda C, Tajima T, Kuma K, Miyauchi A, Ieiri T (2006) Haplotype analysis reveals founder effects of thyroglobulin gene mutations C1058R and C1977S in Japan. J Clin Endocrinol Metab 91:3100–3104
Hishinuma A, Fukata S, Kakudo K, Murata Y, Ieiri T (2005) High incidence of thyroid cancer in long-standing goiters with thyroglobulin mutations. Thyroid 15:1079–1084
Perez-Centeno C, Gonzalez-Sarmiento R, Mories MT, Corrales JJ, Miralles-Garcia JM (1996) Thyroglobulin exon 10 gene point mutation in a patient with endemic goiter. Thyroid 6:423–427
Shim JH, Lee Y (2009) Amperometric nitric oxide microsensor based on nanopore-platinized platinum: the application for imaging NO concentrations. Anal Chem 81:8571–8576
Jung HS, Komatsu S, Ikebe M, Craig R (2008) Head-head and head-tail interaction: a general mechanism for switching off myosin II activity in cells. Mol Biol Cell 19:3234–3242
Schuck P (2000) Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys J 78:1606–1619
Berg G, Bjorkman U, Ekholm R (1980) Conformational change of the thyroglobulin molecule induced by oxidation in vitro. Mol Cell Endocrinol 17:139–144
Hoyer W, Antony T, Cherny D, Heim G, Jovin TM, Subramaniam V (2002) Dependence of alpha-synuclein aggregate morphology on solution conditions. J Mol Biol 322:383–393
Zurdo J, Guijarro JI, Jimenez JL, Saibil HR, Dobson CM (2001) Dependence on solution conditions of aggregation and amyloid formation by an SH3 domain. J Mol Biol 311:325–340
Dunn AD, Corsi CM, Myers HE, Dunn JT (1998) Tyrosine 130 is an important outer ring donor for thyroxine formation in thyroglobulin. J Biol Chem 273:25223–25229
Marriq C, Lejeune PJ, Venot N, Vinet L (1991) Hormone formation in the isolated fragment 1-171 of human thyroglobulin involves the couple tyrosine 5 and tyrosine 130. Mol Cell Endocrinol 81:155–164
Baudry N, Lejeune PJ, Delom F, Vinet L, Carayon P, Mallet B (1998) Role of multimerized porcine thyroglobulin in iodine storage. Biochem Biophys Res Commun 242:292–296
Delom F, Lejeune PJ, Vinet L, Carayon P, Mallet B (1999) Involvement of oxidative reactions and extracellular protein chaperones in the rescue of misassembled thyroglobulin in the follicular lumen. Biochem Biophys Res Commun 255:438–443
Herzog V, Berndorfer U, Saber Y (1992) Isolation of insoluble secretory product from bovine thyroid: extracellular storage of thyroglobulin in covalently cross-linked form. J Cell Biol 118:1071–1083
Saber-Lichtenberg Y, Brix K, Schmitz A, Heuser JE, Wilson JH, Lorand L, Herzog V (2000) Covalent cross-linking of secreted bovine thyroglobulin by transglutaminase. FASEB J 14:1005–1014
Liu XW, Sok DE (2002) Role of protein disulfide isomerase in molecular fate of thyroglobulin and its regulation by endogenous oxidants and reductants. Arch Pharm Res 25:485–492
Esteves RZ, van Sande J, Dumont JE (1992) Nitric oxide as a signal in thyroid. Mol Cell Endocrinol 90:R1–R3
Derakhshan B, Hao G, Gross SS (2007) Balancing reactivity against selectivity: the evolution of protein S-nitrosylation as an effector of cell signaling by nitric oxide. Cardiovasc Res 75:210–219
Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS (2005) Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6:150–166
Schopfer FJ, Baker PR, Freeman BA (2003) NO-dependent protein nitration: a cell signaling event or an oxidative inflammatory response? Trends Biochem Sci 28:646–654
Acknowledgments
We thank Seong Oak Park for EM imaging. AUC performed at the Central laboratory of Mokpo National University. This work was supported by the Converging Research Center Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012K001403) and by Korea Basic Science Institute Grant (T33415).
Conflict of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
You, DJ., Jhon, GJ. & Jung, H.S. Molecular Assembly of Thyroglobulin Induced by In Vitro Nitric Oxide Treatments: Implication Its Role in Thyroid Cells. Protein J 32, 619–625 (2013). https://doi.org/10.1007/s10930-013-9524-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-013-9524-z