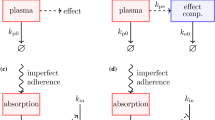
Abstract
Medication nonadherence is one of the largest problems in healthcare today, particularly for patients undergoing long-term pharmacotherapy. To combat nonadherence, it is often recommended to prescribe so-called “forgiving” drugs, which maintain their effect despite lapses in patient adherence. Nevertheless, drug forgiveness is difficult to quantify and compare between different drugs. In this paper, we construct and analyze a stochastic pharmacokinetic/pharmacodynamic (PK/PD) model to quantify and understand drug forgiveness. The model parameterizes a medication merely by an effective rate of onset of effect when the medication is taken (on-rate) and an effective rate of loss of effect when a dose is missed (off-rate). Patient dosing is modeled by a stochastic process that allows for correlations in missed doses. We analyze this “on/off” model and derive explicit formulas that show how treatment efficacy depends on drug parameters and patient adherence. As a case study, we compare the effects of nonadherence on the efficacy of various antihypertensive medications. Our analysis shows how different drugs can have identical efficacies under perfect adherence, but vastly different efficacies for adherence patterns typical of actual patients. We further demonstrate that complex PK/PD models can indeed be parameterized in terms of effective on-rates and off-rates. Finally, we have created an online app to allow pharmacometricians to explore the implications of our model and analysis.






Similar content being viewed by others
References
Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, Dobbels F, Fargher E, Morrison V, Lewek P et al (2012) A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 73(5):691–705
Osterberg L, Blaschke T (2005) Adherence to medication. N Engl J Med 353(5):487–497
Kim J, Combs K, Downs J, Tillman F III (2018) Medication adherence: the elephant in the room. US Pharmacist 43:30–34
De Geest S, Zullig LL, Dunbar-Jacob J, Helmy R, Hughes DA, Wilson IB, Vrijens B (2018) ESPACOMP medication adherence reporting guideline (EMERGE). Ann Intern Med 169(1):30–35
Lowy A, Munk VC, Ong SH, Burnier M, Vrijens B, Tousset EP, Urquhart J (2011) Effects on blood pressure and cardiovascular risk of variations in patients’ adherence to prescribed antihypertensive drugs: role of duration of drug action. Int J Clin Pract 65(1):41–53
Stauffer ME, Hutson P, Kaufman AS, Morrison A (2017) The adherence rate threshold is drug specific. Drugs R &D 17(4):645–653
Assawasuwannakit P, Braund R, Duffull SB (2015) Quantification of the forgiveness of drugs to imperfect adherence. CPT 4(3):204–211
Assawasuwannakit P, Braund R, Duffull SB (2016) A framework for quantifying the influence of adherence and dose individualization. Clin Pharmacol Ther 99(4):452–459
Boissel J-P, Nony P (2002) Using pharmacokinetic-pharmacodynamic relationships to predict the effect of poor compliance. Clin Pharmacokinet 41(1):1–6
Garnett WR, McLean AM, Zhang Y, Clausen S, Tulloch SJ (2003) Simulation of the effect of patient nonadherence on plasma concentrations of carbamazepine from twice-daily extended-release capsules. Curr Med Res Opin 19(6):519–525
Reed RC, Dutta S (2004) Predicted serum valproic acid concentrations in patients missing and replacing a dose of extended-release divalproex sodium. Am J Health Syst Pharm 61(21):2284–2289
Dutta S, Reed RC (2006) Effect of delayed and/or missed enteric-coated divalproex doses on valproic acid concentrations: simulation and dose replacement recommendations for the clinician 1. J Clin Pharm Ther 31(4):321–329
Ding J, Zhang Y, Jiao Z, Wang Y (2012) The effect of poor compliance on the pharmacokinetics of carbamazepine and its epoxide metabolite using monte carlo simulation. Acta Pharmacol Sin 33(11):1431–1440
Chen C, Wright J, Gidal B, Messenheimer J (2013) Assessing impact of real-world dosing irregularities with lamotrigine extended-release and immediate-release formulations by pharmacokinetic simulation. Ther Drug Monit 35(2):188–193
Gidal BE, Majid O, Ferry J, Hussein Z, Yang H, Zhu J, Fain R, Laurenza A (2014) The practical impact of altered dosing on perampanel plasma concentrations: pharmacokinetic modeling from clinical studies. Epilepsy Behav 35:6–12
Brittain ST, Wheless JW (2015) Pharmacokinetic simulations of topiramate plasma concentrations following dosing irregularities with extended-release vs. immediate-release formulations. Epilepsy Behav 52:31–36
Sunkaraneni S, Blum D, Ludwig E, Chudasama V, Fiedler-Kelly J, Marvanova M, Bainbridge J, Phillips L (2018) Population pharmacokinetic evaluation and missed-dose simulations for eslicarbazepine acetate monotherapy in patients with partial-onset seizures. Clin Pharmacol Drug Dev 7(3):287–297
Hard ML, Wehr AY, Sadler BM, Mills RJ, von Moltke L (2018) Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet 43(4):461–469
Elkomy MH (2020) Changing the drug delivery system: does it add to non-compliance ramifications control? A simulation study on the pharmacokinetics and pharmacodynamics of atypical antipsychotic drug. Pharmaceutics 12(4):297
Jia-qin G, Guo Y, Jiao Z, Ding J, Li G-F (2020) How to handle delayed or missed doses: a population pharmacokinetic perspective. Eur J Drug Metab Pharmacokinet 45(2):163–172
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn. Marcelly Dekker, New York
Felmlee MA, Morris ME, Mager DE. Mechanism-based pharmacodynamic modeling. In: Computational toxicology, pp 583–600. Springer, Berlin (2012)
Osterberg LG, Urquhart J, Blaschke TF (2010) Understanding forgiveness: minding and mining the gaps between pharmacokinetics and therapeutics. Clin Pharmacol Ther 88(4):457–459
Urquhart J (2002) The odds of the three nons when an aptly prescribed medicine isn’t working: non-compliance, non-absorption, non-response. Br J Clin Pharmacol 54(2):212–220
Levy G (1993) A pharmacokinetic perspective on medicament noncompliance. Clin Pharmacol Ther 54(3):242–244
Web app at https://seanlawley.shinyapps.io/OnOff/
Norris JR (1998) Markov chains. In: Statistical & probabilistic mathematics. Cambridge University Press, Cambridge
Yuval P, Wilhelm S, Boris S (2000) Sixty years of Bernoulli convolutions. In: Bandt C, Graf S, Zähle M (eds) Fractal geometry and stochastics II. Birkhäuser, Basel, pp 39–65
Solomyak B (1995) On the random series \(\sum \pm \lambda ^n\) (an erdos problem). Ann Math 142(3):611–625
Escribano C, Sastre MA, Torrano E (2001) Moments of infinite convolutions of symmetric Bernoulli distributions. J Comput Appl Math 153(1):191–199, 2003. In: Proceedings of the 6th international symposium on orthogonal polynomials, special functions and their applications, Rome, Italy, 18–22
Tian-You H, Lau K-S (2008) Spectral property of the Bernoulli convolutions. Adv Math 219(2):554–567
Kershner R, Wintner A (1935) On symmetric Bernoulli convolutions. Am J Math 57(3):541–548
Jessen B, Wintner A (1935) Distribution functions and the Riemann zeta function. Trans Am Math Soc 38(1):48–88
Wintner A (1935) On symmetric Bernoulli convolutions. Bull Am Math Soc 41:137–138
Erdös P (1939) On a family of symmetric Bernoulli convolutions. Am J Math 61:974
Lévy-Véhel P-E, Lévy-Véhel J (2013) Variability and singularity arising from poor compliance in a pharmacokinetic model I: the multi-IV case. J Pharmacokinet Pharmacodyn 40(1):15–39
Fermín LJ, Lévy-Véhel J (2017) Variability and singularity arising from poor compliance in a pharmacokinetic model II: the multi-oral case. J Math Biol 74(4):809–841
Counterman ED, Lawley SD (2021) What should patients do if they miss a dose of medication? A theoretical approach. J Pharmacokinet Pharmacodyn 48(6):873–892
Counterman ED, Lawley SD (2022) Designing drug regimens that mitigate nonadherence. Bull Math Biol 84(1):1–36
Burnier M (2019) Is there a threshold for medication adherence? Lessons learnt from electronic monitoring of drug adherence. Front Pharmacol 9:1540
Brian HR (1976) Compliance with therapeutic regimens, chapter A critical review of “determinants’’ of patient compliance with therapeutic regimens. Johns Hopkins University Press, Baltimore, pp 26–39
Radauceanu A, Virion J-M, Boivin J-M, Zannad F (2002) Time-effect profile of antihypertensive agents assessed with trough/peak ratio, smoothness index and dose omission: an ambulatory blood pressure monitoring study with trandolapril vs. quinapril1. Fundam Clin Pharmacol 16(6):545–554
Vaur L, Bobrie G, Dutrey-Dupagne C, Dubroca I, Vaisse B, Bouvier d’Yvoire M, Elkik FE, Chatellier G, Menard J (1998) Short-term effects of withdrawing angiotensin converting enzyme inhibitor therapy on home self-measured blood pressure in hypertensive patients. Am J Hypertens 11(2):165–173
Palatini P, Jung W, Shlyakhto E, Botha J, Bush C, Keefe DL (2010) Maintenance of blood-pressure-lowering effect following a missed dose of aliskiren, irbesartan or ramipril: results of a randomized, double-blind study. J Hum Hypertens 24(2):93–103
Yves Lacourciere and for the Candesartan/Losartan study investigators Asmar, Roland. A comparison of the efficacy and duration of action of candesartan cilexetil and losartan as assessed by clinic and ambulatory blood pressure after a missed dose, in truly hypertensive patients*: a placebo-controlled, forced titration study. Am J Hypertens 12(12):1181–1187 (1999)
Johnson BF, Whelton A (1994) A study design for comparing the effects of missing daily doses of antihypertensive drugs. Am J Ther 1(4):260–267
Smilde JG (1997) A comparison of amlodipine and felodipine extended release in the treatment of hypertension at steady state and after two missed doses. Curr Ther Res 58(3):141–153
Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M (2008) Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 336(7653):1114–1117
AHFS DI Essentials. Warfarin. https://www.drugs.com/monograph/warfarin.html. Accessed 8 Feb 2023
Lixoft SAS, a Simulations Plus company. Monolix 2021r2. https://lixoft.com/products/monolix/
Lixoft SAS, a Simulations Plus company. Case studes. https://monolix.lixoft.com/case-studies/. Accessed 8 Feb 2023
O’Reilly RA, Aggeler PM (1968) Studies on coumarin anticoagulant drugs. Circulation 38(1):169–177
Waterman AD, Milligan PE, Bayer L, Banet GA, Gatchel SK, Gage BF (2004) Effect of warfarin nonadherence on control of the International Normalized Ratio. Am J Health Syst Pharm 61(12):1258–1264
Kimmel SE, Chen Z, Price M, Parker CS, Metlay JP, Christie JD, Brensinger CM, Newcomb CW, Samaha FF, Gross R (2007) The influence of patient adherence on anticoagulation control with warfarin: results from the international normalized ratio adherence and genetics (IN-RANGE) study. Arch Intern Med 167(3):229–235
Platt AB, Localio AR, Brensinger CM, Cruess DG, Christie JD, Gross R, Parker CS, Price M, Metlay JP, Cohen A, Newcomb CW, Strom BL, Laskin MS, Kimmel SE (2008) Risk factors for nonadherence to warfarin: results from the in-range study. Pharmacoepidemiol Drug Saf 17(9):853–860
Yao X, Abraham NS, Alexander GC, Crown W, Montori VM, Sangaralingham LR, Gersh BJ, Shah ND, Noseworthy PA (2016) Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc 5(2):e003074
AHFS DI Essentials. Metformin. Data retrieved on 8 February 2023 from https://www.drugs.com/monograph/metformin.html
Hong Y, Rohatagi S, Habtemariam B, Walker JR, Schwartz SL, Mager DE (2008) Population exposure-response modeling of metformin in patients with type 2 diabetes mellitus. J Clin Pharmacol 48(6):696–707
McAllister N, Lawley S (2022) A pharmacokinetic and pharmacodynamic analysis of drug forgiveness. J Pharmacokinet Pharmacodyn 49:1–17
Tseng Y-J, Steinberg G, Fox KP, Armstrong J, Mandl KD (2017) Antihyperglycemic medications: a claims-based estimate of first-line therapy use prior to initialization of second-line medications. Diabetes Care 40(11):1500–1505
Parente M, Pignata I, Gnavi R, Spadea T, Di Martino M, Baratta F, Brusa P (2020) Assessment of non-adherence to oral metformin and atorvastatin therapies: a cross-sectional survey in piedmont (Italy). Patient Prefer Adherence 14:261–266
Christofides EA (2019) Practical insights into improving adherence to metformin therapy in patients with type 2 diabetes. Clin Diabetes 37(3):234–241
McGovern A, Tippu Z, Hinton W, Munro N, Whyte M, de Lusignan S (2018) Comparison of medication adherence and persistence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 20(4):1040–1043
Sun J, Nagaraj HN, Reynolds NR (2007) Discrete stochastic models for compliance analysis based on an AIDS clinical trial group (ACTG) study. Biom J 49(5):731–741
Hammer SM, Vaida F, Bennett KK, Holohan MK, Sheiner L, Eron JJ, Wheat LJ, Mitsuyasu RT, Gulick RM, Valentine FT et al (2002) Dual vs single protease inhibitor therapy following antiretroviral treatment failure: a randomized trial. JAMA 288(2):169–180
Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS et al (2014) Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid 24(12):1670–1751
Clark ED, Lawley SD (2022) Should patients skip late doses of medication? A pharmacokinetic perspective. J Pharmacokinet Pharmacodyn 49(4):429–444
Acknowledgements
This work was supported by the National Science Foundation (Grant Nos. DMS-1944574 and DMS-1814832).
Author information
Authors and Affiliations
Contributions
EDC and SDL jointly devised and carried out the research and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
In this Appendix, we collect details of the mathematical analysis.
Moments of drug effect
We now compute the first and second moments in (7)–(8). First, for the transition probabilities in (4)–(5), the transition matrix is
and the stationary distribution is
Hence, the proportion of doses taken is
Note that the first moment of X is given by the sum
where \(\mathbbm {1}_{E}\in \{0,1\}\) denotes the indicator function, which takes on a value of 1 if the event E occurs and 0 otherwise. At steady state (i.e. the patient has been taking the medication for sufficiently long at adherence rate p), we have
where \(X{\mathop {=}\limits ^{\text {d}}}Y\) denotes equality in distribution. Therefore,
which means that
Taking the expected value, we have
where \(\pi (1) = p\). Note that
where the second equality comes from the tower property of conditional expectation. The term within the sum can be simplified. For a function \(f(X_{0},j)\),
where \(P_{ij}\) is the ij-th entry of the transition matrix (20). This means that
Combining this result with (22) we have
These lead to the system of equations
The solution to this system is
Thus,
The second moment can be computed in a similar fashion. Note that
and thus
The results in (23) and (24) can be applied to \({\mathbb {E}}[X_{0}^2\mathbbm {1}_{\xi _1=j}]\), which gives
Thus,
From this system of equations we have
which means that the second moment is
Numerical computation of \({\mathbb {P}}(X>\theta )\)
Assuming independence of \(\{\xi _{n}\}_{n\in {\mathbb {Z}}}\), the distribution function of the biomarker response level can be numerically computed using the recursion relationship (3). By (21), we have that
Denoting the distribution function of X by \(F(x) = {\mathbb {P}}(X \le x)\), we thus have
By starting with an initial guess for the distribution function of X and iteratively applying (26), the true distribution function of X can be numerically determined. Specifically, we obtain a sequence of approximating distribution functions \(\{F_{m}(x)\}_{m\ge 0}\) where \(F_{0}(x)=x\) and \(F_{m+1}(x)\) is defined by \(F_{m}(x)\) via
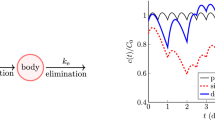
The dashed curves in Fig. 4 are obtained via this recursion once successive iterations \(F_{m}(x)\) and \(F_{m+1}(x)\) differ by less than \(10^{-10}\) for all \(x\in [0,1]\).
Beta distribution formulas
In the "Mean and variance of biomarker response" section, we described how we can approximate the full probability distribution of X by a Beta random variable B chosen so that the first and second moments of X and B agree. We now give the explicit formulas for this Beta distribution fit.
The probability density function of a Beta random variable B is given by
and \(f_{B}(x)=0\) for \(x\notin (0,1)\), where \(\Gamma (z)=\int _{0}^{\infty }u^{z-1}e^{-u}\,\text {d} u\) denotes the Gamma function. The first and second moments of B are
Therefore, choosing a and b so that \({\mathbb {E}}[B]={\mathbb {E}}[X]\) and \({\mathbb {E}}[B^{2}]={\mathbb {E}}[X^{2}]\) implies
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Clark, E.D., Lawley, S.D. How drug onset rate and duration of action affect drug forgiveness. J Pharmacokinet Pharmacodyn (2024). https://doi.org/10.1007/s10928-023-09897-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10928-023-09897-1