Abstract
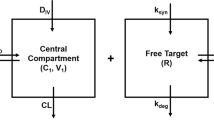
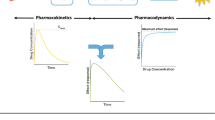
Predictions for target engagement are often used to guide drug development. In particular, when selecting the recommended phase 2 dose of a drug that is very safe, and where good biomarkers for response may not exist (e.g. in immuno-oncology), a receptor occupancy prediction could even be the main determinant in justifying the approved dose, as was the case for atezolizumab. The underlying assumption in these models is that when the drug binds its target, it disrupts the interaction between the target and its endogenous ligand, thereby disrupting downstream signaling. However, the interaction between the target and its endogenous binding partner is almost never included in the model. In this work, we take a deeper look at the in vivo system where a drug binds to its target and disrupts the target’s interaction with an endogenous ligand. We derive two simple steady state inhibition metrics (SSIMs) for the system, which provides intuition for when the competition between drug and endogenous ligand should be taken into account for guiding drug development.












Similar content being viewed by others
References
Deng R, Bumbaca D, Pastuskovas CV, Boswell CA, West D, Cowan KJ, Chiu H, McBride J, Johnson C, Xin Y et al (2016) Preclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-pd-l1 monoclonal antibody, an immune checkpoint inhibitor. MAbs 8(3):593–603
Fu W, Liu C, Dorff S, Liu Q, Yu J, and Charlab OR (2016) Clinical pharmacology and biopharmaceutics review for atelolizumab in locally advanced or metastatic urothelial carcinoma 761034Orig1s000. Center for Drug Evaluation Research
Stein A, Ramakrishna R (2017) Afir: A dimensionless potency metric for characterizing the activity of monoclonal antibodies. Pharmacomet Syst Pharmacol 6(4):258–266
Ahmed S, Ellis M, Li H, Pallucchini L, Stein AM (2019) Guiding dose selection of monoclonal antibodies using a new parameter (aftir) for characterizing ligand binding systems. J Pharmacokinet Pharmacodyn 46(3):287–304
DeWitt AE, Dong JY, Wiley HS, Lauffenburger DA (2001) Quantitative analysis of the egf receptor autocrine system reveals cryptic regulation of cell response by ligand capture. J Cell Sci 114(12):2301–2313
DeWitt A, Iida T, Lam H-Y, Hill V, Wiley HS, Lauffenburger DA (2002) Affinity regulates spatial range of egf receptor autocrine ligand binding. Developmental biology 250(2):305–316
Janes KA, Gaudet S, Albeck JG, Nielsen UB, Lauffenburger DA, Sorger PK (2006) The response of human epithelial cells to tnf involves an inducible autocrine cascade. Cell 124(6):1225–1239
Yan X, Chen Y, Krzyzanski W (2012) Methods of solving rapid binding target-mediated drug disposition model for two drugs competing for the same receptor. J Pharmacokinet Pharmacodyn 39(5):543–560
Koch G et al (2017) Target mediated drug disposition with drug-drug interaction, part ii: competitive and uncompetitive cases. J Pharmacokinet Pharmacodyn
Wu N, Hammock B, Lee KSS, An G (2020) Simultaneous target-mediated drug disposition (tmdd) model for two small-molecule compounds competing for their pharmacological target: Soluble epoxide hydrolase. J Pharmacol Exp Ther
Peletier LA, Benson N, van der Graaf PH (2009) Impact of plasma-protein binding on receptor occupancy: an analytical description. J Theor Biol 256(2):253–262
Peletier LA, Gabrielsson J (2018) New equilibrium models of drug-receptor interactions derived from target-mediated drug disposition. AAPS J 20(4):69
Gibiansky L, Gibiansky E (2009) Target-mediated drug disposition model: approximations, identifiability of model parameters and applications to the population pharmacokinetic-pharmacodynamic modeling of biologics. Expert Opin Drug Metab Toxicol 5(7):803–812
Ma P (2012) Theoretical considerations of target-mediated drug disposition models: simplifications and approximations. Pharm Res 29(3):866–882
Krippendorff B-F, Kuester K, Kloft C, Huisinga W (2009) Nonlinear pharmacokinetics of therapeutic proteins resulting from receptor mediated endocytosis. J Pharmacokinet Pharmacodyn 36(3):239–260
Knutson V (1992) Ligand-independent internalization and recycling of the insulin receptor. effects of chronic treatment of 3t3-c2 fibroblasts with insulin and dexamethasone. J Biol Chem 267(2):931–937
Page KR, Mezzalana E, MacDonald AJ, Zamuner S, De Nicolao G, van Maurik A (2015) Temporal pharmacokinetic/pharmacodynamic interaction between human cd3\(\varepsilon\) antigen-targeted monoclonal antibody otelixizumab and cd3\(\varepsilon\) binding and expression in human peripheral blood mononuclear cell static culture. J Pharmacol Exp Ther 355(2):199–205
Gabrielsson J, Peletier LA (2017) Pharmacokinetic steady-states highlight interesting target-mediated disposition properties. AAPS J 19(3):772–786
Fujimoto K, Ida H, Hirota Y, Ishigai M, Amano J, Tanaka Y (2015) Intracellular dynamics and fate of a humanized anti-interleukin-6 receptor monoclonal antibody, tocilizumab. Mol Pharmacol 88(4):660–675
Gibiansky L, Frey N (2012) Linking interleukin-6 receptor blockade with tocilizumab and its hematological effects using a modeling approach. J Pharmacokinet Pharmacodyn 39(1):5–16
Gaddum JH (1937) The quantitative effects of antagonistic drugs. J Physiol 89:7P–9P
Stein AM, Peletier LA (2018) Predicting the onset of nonlinear pharmacokinetics. CPT 7(10):670–677
Dirks NL, Meibohm B (2010) Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 49(10):633–659
Yang D, Singh A, Wu H, Kroe-Barrett R (2016) Comparison of biosensor platforms in the evaluation of high affinity antibody-antigen binding kinetics. Anal Biochem 508:78–96
Charoin J-E, Levi M, Frey N, Delor I, and Jacqmin P (2010) Target mediated drug disposition (TMDD) in rheumatoid arthritis, a case study using tocilizumab. In: Presenetd at the 6th International Symposium on Measurement and Kinetics of in Vivo Drug Effects in Noordwijkerhout, The Netherlands
Rowland M and Tozer T (2011) Clinical pharmacokinetics and pharmacodynamics concepts and applications, 4th edn. Wolters Kluwer
Jm B, Jl T, L S, (2002) Biochemistry, 5th edn. W H Freeman, New York
Zheng S, McIntosh T, Wang W (2015) Utility of free and total target measurements as target engagement and efficacy biomarkers in biotherapeutic development–opportunities and challenges. J Clin Pharmacol 55(S3):S75–S84
Kim J, Khanshan F, Ho Y-Y, Stein A (2018) Utilizing receptor occupancy and tumor penetration for the phase 2 dose selection of monoclonal antibodies targeting solid tumors. J Pharmacokinet Pharmacodyn 45:S49–S49
Acknowledgements
We would like to thank Mirjam Trame for co-organizing the 2018 Novartis-Academia Quantitative Hackathon, where this work began and we would also like to thank our other Hackathon team members, Ryan Richards and Ruidi Chen. In addition, Matt Fidler helped with RxODE for the model simulations; Khachik Sargasyan helped us think through how to perform the global sensitivity analysis; Bert Peletier helped with the organizational structure of this manuscript; Martin Fink helped with clarifying the key message and model description; and Jaeyeon Kim helped with parameter selection and thinking through the implications of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alaybeyoglu, B., Cheng, H.W.(., Doshi, K.A. et al. Estimating drug potency in the competitive target mediated drug disposition (TMDD) system when the endogenous ligand is included.. J Pharmacokinet Pharmacodyn 48, 447–464 (2021). https://doi.org/10.1007/s10928-020-09734-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-020-09734-9