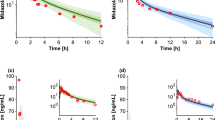
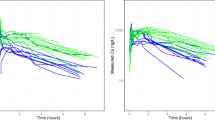
Abstract
The effect of obesity on the shape of drug disposition curves was explained using the residence time concept without assuming well-mixed compartments. The mean (MDRT) and relative dispersion \({({\rm RD}_{\rm D}^2)}\) of disposition residence time of drug were predicted as function of percentage body fat by lumping the organs into fat and nonfat tissues, utilizing the fact that MDRT and \({{\rm RD}_{\rm D}^2}\) act as a scale and shape parameter of disposition curves, respectively. The longer sojourn time of lipophilic drugs in adipose tissue leads to an increase in \({{\rm RD}_{\rm D}^2}\) when the fraction of body fat increases. This explains the change in the shape of disposition curves observed in obese patients, where the increase in MDRT is accompanied by a proportionately great prolongation of the terminal half life. The model also predicts a decrease in whole body distribution clearance with increasing residence time dispersion \({({\rm RD}_{\rm D}^2)}\) .
Similar content being viewed by others
Abbreviations
- \({C_{\rm D}(t)}\) :
-
Drug disposition curve after i.v. bolus injection
- MDRT:
-
Mean disposition residence time
- VDRT:
-
Variance of disposition residence time
- \({{\rm RD}_{\rm D}^2}\) :
-
Relative dispersion of disposition residence time
- \({{\rm RD}_{\rm C}^2}\) :
-
Relative dispersion of circulatory transit time
- \({{\rm RD}_{\rm B}^2}\) :
-
Relative dispersion of vascular transit time
- CL:
-
Total elimination clearance
- \({{\widetilde{\rm CL}}}\) :
-
CL/(Body weight) = CL/V body
- CLM :
-
Whole body distribution clearance
- Q :
-
Cardiac output
- i = F:
-
Fat tissue
- i = NF:
-
Nonfat tissue
- Q i :
-
Blood flow to tissue i
- qF:
-
\({Q_{\rm F}/Q}\)
- E :
-
Total extraction ratio (= CL/Q)
- \({K_{{\rm p},i}}\) :
-
Tissue:plasma partition coefficient of tissue i
- \({k_{\rm F}}\) :
-
\({K_{{\rm p,F}}/K_{{\rm p,NF}}}\)
- V i :
-
Physiological volume of tissue i
- bf:
-
\({V_{\rm F}/V_{\rm body}}\)
- V ss :
-
Volume of distribution at steady-state
- \({\tilde{V}_{\rm ss}}\) :
-
\({V_{\rm ss}/V_{\rm body}}\)
- V z :
-
Terminal distribution volume
- t 1/2,z :
-
Terminal half-live
References
Stein CJ, Colditz GA (2004) The epidemic of obesity. J Clin Endocrinol Metab 89: 2522–2525
Blouin RA, Warren GW (1999) Pharmacokinetic considerations in obesity. J Pharm Sci 88: 1–7
Cheymol G (2000) Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet 39: 215–231
Bouillon T, Shafer SL (1998) Does size matter. Anesthesiology 89: 557–560
Casati A, Putzu M (2005) Anesthesia in the obese patient: pharmacokinetic considerations. J Clin Anesth 17: 134–145
Brodie BB, Bernstein E, Mark LC (1952) The role of body fat in limiting the duration of action of thiopental. J Pharmacol Exp Ther 105: 421–426
Jung D, Mayersohn M, Perrier D, Calkins J, Saunders R (1982) Thiopental disposition in lean and obese patients undergoing surgery. Anesthesiology 56: 269–274
Russo H, Bressolle F (1998) Pharmacodynamics and pharmacokinetics of thiopental. Clin Pharmacokinet 35: 95–134
Wada DR, Bjorkman S, Ebling WF, Harashima H, Harapat SR, Stanski DR (1997) Computer simulation of the effects of alterations in blood flows and body composition on thiopental pharmacokinetics in humans. Anesthesiology 87: 884–899
Weiss M, Krejcie TC, Avram MJ (2007) A minimal physiological model of thiopental distribution kinetics based on a multiple indicator approach. Drug Metab Disp 35: 1525–1532
Weiss M (1986) Generalizations in linear pharmacokinetics using properties of certain classes of residence time distributions. I. Log-convex drug disposition curves. J Pharmacokinet Biopharm 14: 635–657
Weiss M (1992) The relevance of residence time theory to pharmacokinetics. Eur J Clin Pharmacol 43: 571–579
Weiss M (2007) Residence time dispersion as a general measure of drug distribution kinetics: estimation and physiological interpretation. Pharm Res 24: 2025–2030
Weiss M, Krejcie TC, Avram MJ (2006) Transit time dispersion in the pulmonary and systemic circulation: effects of cardiac output and solute diffusivity. Am J Physiol Heart Circ Physiol 291: H861–870
Bjorkman S (2002) Prediction of the volume of distribution of a drug: which tissue–plasma partition coefficients are needed. J Pharm Pharmacol 54: 1237–1245
Weiss M (1983) Hemodynamic influences upon the variance of disposition residence time distribution of drugs. J Pharmacokinet Biopharm 11: 63–75
Weiss M (1995) Distribution kinetics in the body and single organs: moment analysis. In: D’Argenio DZ(eds) Advanced methods of pharmacokinetic and pharmacodynamic system analysis. Plenum Press, New York, pp 89–100
Weiss M, Krejcie TC, Avram MJ (2007) Circulatory transport and capillary-tissue exchange as determinants of the distribution kinetics of inulin and antipyrine in dog. J Pharm Sci 96: 913–926
Weiss M (1983) Use of gamma distributed residence times in pharmacokinetics. Eur J Clin Pharmacol 25: 695–702
PriorBM, Cureton KJ, Modlesky CM, Evans EM, Sloniger MA, Saunders M, Lewis RD (1997) In vivo validation of whole body composition estimates from dual-energy X-ray absorptiometry. J Appl Physiol 83: 623–630
Collis T, Devereux RB, Roman MJ, de Simone G, Yeh J, Howard BV, Fabsitz RR, Welty TK (2001) Relations of stroke volume and cardiac output to body composition: the strong heart study. Circulation 103: 820–825
Kabon B, Nagele A, Reddy D, Eagon C, Fleshman JW, Sessler DI, Kurz A (2004) Obesity decreases perioperative tissue oxygenation. Anesthesiology 100: 274–280
Coppack SW (2005) Adipose tissue changes in obesity. Biochem Soc Trans 33: 1049–1052
Ebling WF, Wada DR, Stanski DR (1994) From piecewise to full physiologic pharmacokinetic modeling: applied to thiopental disposition in the rat. J Pharmacokinet Biopharm 22: 259–292
D’Argenio DZ, Schumitzky A (1997) ADAPT II User’s guide: pharmacokinetic pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles
Deurenberg P, Yap M, van Staveren WA (1998) Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord 22: 1164–1171
Greenblatt DJ, Abernethy DR, Locniskar A, Harmatz JS, Limjuco RA, Shader RI (1984) Effect of age, gender, and obesity on midazolam kinetics. Anesthesiology 61: 27–35
Steiner SH, Moor MJ, Bickel MH (1991) Kinetics of distribution and adipose tissue storage as a function of lipophilicity and chemical structure.. I. Barbiturates Drug Metab Dispos 19: 8–14
Poulin P, Schoenlein K, Theil FP (2001) Prediction of adipose tissue: plasma partition coefficients for structurally unrelated drugs. J Pharm Sci 90: 436–447
Mclean AJ, Le Couteur DG (2004) Aging biology and geriatric clinical pharmacology. Pharmacol Rev 56: 163–184
Krejcie TC, Avram MJ (1999) What determines anesthetic induction dose? It’s the front-end kinetics, doctor!. Anesth Analg 89: 541–544
Avram MJ, Krejcie TC (2003) Using front-end kinetics to optimize target-controlled drug infusions. Anesthesiology 99: 1078–1086
De Baerdemaeker LEC, Mortier EP, Struys MMRF (2004) Pharmacokinetic in obese patients. Contin Educ Anaesth Crit Care Pain 4: 152–155
Turcant A, Delhumeau A, Premel-Cabic A, Granry JC, Cottineau C, Six P, Allain P (1985) Thiopental pharmacokinetics under conditions of long-term infusion. Anesthesiology 63: 50–54
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weiss, M. How does obesity affect residence time dispersion and the shape of drug disposition curves? Thiopental as an example. J Pharmacokinet Pharmacodyn 35, 325–336 (2008). https://doi.org/10.1007/s10928-008-9090-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-008-9090-8