Abstract
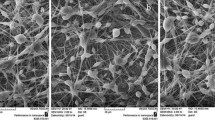
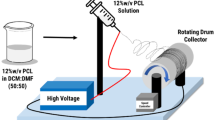
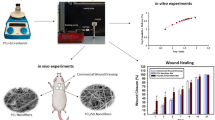
Alkaline pH levels in chronic wounds increase the risk of bacterial accumulation. Therefore, the pH value is a key factor in the wound healing process. In this regard, an attempt was made to introduce a new composite nanofiber for the treatment of chronic wounds. In this wound dressing, the composite of Eudragit® L-100 (EU), which dissolves only at pH above 6, and hydroxypropyl methyl cellulose (EU/HPMC) was used to load propolis (PRO), which has antibacterial and antioxidant properties. We investigated the morphological and physicochemical properties of this new pH-sensitive mat composed of different blending ratios of EU/HPMC/PRO. The scanning electron microscope (SEM) images exhibited that by increasing the propolis content in the range of 10–30% v/v, the average diameter of nanofibers increased from 589.82 ± 102.5 to 676.01 ± 127.3 nm. The results of Fourier transform infrared spectroscopy (FT-IR), thermal gravimetric analysis, mechanical properties, and water contact angle of mats confirmed the successful loading of PRO into the nanofibers. In addition, we evaluated the antibacterial properties of optimal mat against Staphylococcus aureus (Gram positive) and Escherichia coli (Gram negative) bacteria, cell proliferation, and cell adhesion. Finally, we compared the release rate of propolis from nanofibers in media at pH 7.4 and 5.5. The results showed that the EU/HPMC/PRO nanofibers had good potential to be used as a wound dressing.














Similar content being viewed by others
References
Lambers H, Piessens S, Bloem A et al (2006) Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci 28:359–370. https://doi.org/10.1111/j.1467-2494.2006.00344.x
Sochorová M, Staňk K, Pullmannová P et al (2017) Permeability barrier and microstructure of skin lipid membrane models of impaired Glucosylceramide Processing. Sci Rep 6470:1–8. https://doi.org/10.1038/s41598-017-06990-7
Sharpe JR, Harris KL, Jubin K et al (2009) The effect of pH in modulating skin cell behaviour. Br J Dermatol 161:671–673. https://doi.org/10.1111/j.1365-2133.2009.09168.x
Alexander L, Andreas S, Grabbe S, Dissemond J (2007) Influence of pH on wound-healing: a new perspective for wound-therapy? Arch Dermatol Res 298:413–420. https://doi.org/10.1007/s00403-006-0713-x
Mostafalu P, Tamayol A, Rahimi R et al (2018) Smart Bandage for Monitoring and Treatment of Chronic Wounds. Small 14:1703509. https://doi.org/10.1002/smll.201703509
Kiaee G, Mostafalu P, Samandari M, Sonkusale S (2018) A pH-Mediated electronic wound dressing for controlled drug delivery. Adv Healthc Mater 7:1800396. https://doi.org/10.1002/adhm.201800396
Mariani F, Sera M, Gualandi I et al (2021) Advanced Wound Dressing for Real-Time pH monitoring. ACS Sens 6:2366–2377. https://doi.org/10.1021/acssensors.1c00552
Kouchak M, Handali S, Naseri B (2015) Evaluation of the Mechanical Properties and Drug Permeability of Chitosan / Eudragit RL Composite Film. Osong Public Heal Res Perspect 6:14–19. https://doi.org/10.1016/j.phrp.2014.12.001
Fan Y, Wu W, Lei Y et al (2019) Edaravone-Loaded Alginate-Based Nanocomposite Hydrogel Accelerated Chronic Wound Healing in Diabetic mice. Mar Drugs 17:285. https://doi.org/10.3390/md17050285
Wittaya-Areekul S, Prahsarn CSS (2006) Development and in Vitro evaluation of Chitosan – Eudragit RS 30D Composite Wound Dressings. AAPS PharmSciTech 7:E215–E220. https://doi.org/10.1208/pt070130
Feleke F, Balzus B, Gerecke C et al (2016) Formulation and in vitro evaluation of polymeric enteric nanoparticles as dermal carriers with pH-dependent targeting potential. Eur J Pharm Sci 92:98–109. https://doi.org/10.1016/j.ejps.2016.07.004
Dong P, Feleke F, Lohan SB et al (2019) pH-sensitive Eudragit ® L 100 nanoparticles promote cutaneous penetration and drug release on the skin. J Control Release 295:214–222. https://doi.org/10.1016/j.jconrel.2018.12.045
Xue J, Xie J, Liu W, Xia Y (2017) Electrospun Nano fi bers: New Concepts, Materials, and Applications. https://doi.org/10.1021/acs.accounts.7b00218
Goyal R, Macri LK, Kaplan HMKJ (2016) Nanoparticles and nano fi bers for topical drug delivery. J Control Release 240:77–92. https://doi.org/10.1016/j.jconrel.2015.10.049
Oryan A, Alemzadeh E, Moshiri A (2018) Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed Pharmacother 98:469–483. https://doi.org/10.1016/j.biopha.2017.12.069
Meimandi-Parizi A, Oryan A, Sayahi E, Bigham-Sadegh A (2018) Propolis extract a new reinforcement material in improving bone healing: an in vivo study. Int J Surg 56:94–101. https://doi.org/10.1016/j.ijsu.2018.06.006
Wagh VD (2013) Propolis: a wonder bees product and its pharmacological potentials. Adv Pharmacol Sci 2013. https://doi.org/10.1155/2013/308249
Braia M, Tubio G, Nerli B et al (2012) Analysis of the interactions between Eudragit ® L100 and porcine pancreatic trypsin by calorimetric techniques. Int J Biol Macromol 50:180–186. https://doi.org/10.1016/j.ijbiomac.2011.10.016
Mohebian Z, Tajmohammadi I, Yavari Maroufi L et al (2022) A Novel Aloe Vera-Loaded Ethylcellulose/Hydroxypropyl methylcellulose nanofibrous mat designed for Wound Healing Application. J Polym Environ 30:867–877. https://doi.org/10.1007/s10924-021-02240-0
Akrami-Hasan-Kohal M, Tayebi L, Ghorbani M (2020) Curcumin-loaded naturally-based nanofibers as active wound dressing mats: morphology, drug release, cell proliferation, and cell adhesion studies. New J Chem 44:10343–10351. https://doi.org/10.1039/D0NJ01594F
Yavari Maroufi L, Ghorbani M, Mohammadi M, Pezeshki A (2021) Improvement of the physico-mechanical properties of antibacterial electrospun poly lactic acid nanofibers by incorporation of guar gum and thyme essential oil. Colloids Surf Physicochem Eng Asp 622:126659. https://doi.org/10.1016/j.colsurfa.2021.126659
Ghorbani M, Nezhad-Mokhtari P, Sohrabi H, Roshangar L (2020) Electrospun chitosan/nanocrystalline cellulose-graft-poly(N-vinylcaprolactam) nanofibers as the reinforced scaffold for tissue engineering. J Mater Sci 55:2176–2185. https://doi.org/10.1007/s10853-019-04115-1
Ghorbani M, Mahmoodzadeh F, Yavari Maroufi L, Nezhad-Mokhtari P (2020) Electrospun tetracycline hydrochloride loaded zein/gum tragacanth/poly lactic acid nanofibers for biomedical application. Int J Biol Macromol 165:1312–1322. https://doi.org/10.1016/j.ijbiomac.2020.09.225
Akrami-Hasan-Kohal M, Ghorbani M, Mahmoodzadeh F, Nikzad B (2020) Development of reinforced aldehyde-modified kappa-carrageenan/gelatin film by incorporation of halloysite nanotubes for biomedical applications. Int J Biol Macromol 160:669–676. https://doi.org/10.1016/j.ijbiomac.2020.05.222
Mohebian Z, Yavari Maroufi L, Ghorbani M (2021) Development of a novel reinforced film based on gellan gum/cellulose nanofiber/soy protein for skin tissue engineering application. New J Chem 45:13814–13821. https://doi.org/10.1039/d1nj02623b
Maroufi LY, Shahabi N, Ghanbarzadeh M, dokht, Ghorbani M (2022) Development of Antimicrobial active food packaging Film based on Gelatin/Dialdehyde quince seed Gum Incorporated with Apple Peel Polyphenols. Food Bioprocess Technol 15:693–705. https://doi.org/10.1016/j.carbpol.2022.119620
Ahmadian S, Ghorbani M, Mahmoodzadeh F (2020) Silver sulfadiazine-loaded electrospun ethyl cellulose/polylactic acid/collagen nanofibrous mats with antibacterial properties for wound healing. Int J Biol Macromol 162:1555–1565. https://doi.org/10.1016/j.ijbiomac.2020.08.059
Ghorbani M, Ramezani S, Rashidi MR (2021) Fabrication of honey-loaded ethylcellulose/gum tragacanth nanofibers as an effective antibacterial wound dressing. Colloids Surf Physicochem Eng Asp 621:126615. https://doi.org/10.1016/j.colsurfa.2021.126615
Maroufi NF, Vahedian V, Mazrakhondi SAM et al (2020) Sensitization of MDA-MBA231 breast cancer cell to docetaxel by myricetin loaded into biocompatible lipid nanoparticles via sub-G1 cell cycle arrest mechanism. Naunyn Schmiedebergs Arch Pharmacol 393:1–11
Raeisi S, Chavoshi H, Mohammadi M et al (2019) Naringenin-loaded nano-structured lipid carrier fortifies oxaliplatin-dependent apoptosis in HT-29 cell line. Process Biochem 83:168–175. https://doi.org/10.1016/j.procbio.2019.05.013
Ghorbani M, Nezhad-Mokhtari P, Ramazani S (2020) Aloe vera-loaded nanofibrous scaffold based on Zein/Polycaprolactone/Collagen for wound healing. Int J Biol Macromol 153:921–930. https://doi.org/10.1016/j.ijbiomac.2020.03.036
Doustdar F, Ghorbani M (2022) ZIF-8 enriched electrospun ethyl cellulose / polyvinylpyrrolidone scaffolds: the key role of polyvinylpyrrolidone molecular weight. Carbohydr Polym 291:119620. https://doi.org/10.1016/j.carbpol.2022.119620
Doustdar F, Ramezani S, Ghorbani M, Moghadam FM (2022) Optimization and characterization of a novel tea tree oil-integrated poly (ε-caprolactone)/soy protein isolate electrospun mat as a wound care system. Int J Pharm 627:122218. https://doi.org/10.1016/j.ijpharm.2022.122218
Bodbodak S, Shahabi N, Mohammadi M et al (2021) Development of a Novel Antimicrobial Electrospun Nanofiber Based on Polylactic Acid/Hydroxypropyl Methylcellulose Containing Pomegranate Peel Extract for Active Food Packaging. Food Bioprocess Technol. https://doi.org/10.1007/s11947-021-02722-y
Marzieh B, Niazmand R (2020) Characterization of polyamide-6 / propolis blended electrospun fi bers. Heliyon 6:e04784. https://doi.org/10.1016/j.heliyon.2020.e04784
Moradkhannejhad L, Abdouss M, Nikfarjam N et al (2018) Electrospinning of zein / propolis nano fi bers; antimicrobial properties and morphology investigation. J Mater Sci Mater Med 29:1–10. https://doi.org/10.1007/s10856-018-6174-x
Mahdavinia GR, Ettehadi S, Amini M, Sabzi M (2015) Synthesis and characterization of hydroxypropyl methylcellulose-g-poly(acrylamide)/LAPONITE® RD nanocomposites as novel magnetic- and pHsensitive carriers for controlled drug release. RSC Adv 5:44516–44523. https://doi.org/10.1039/C5RA03731J
Jena PK, Singh S, Prajapati B et al (2014) Impact of targeted specific antibiotic delivery for Gut Microbiota Modulation on High-Fructose-Fed rats. Appl Biochem Biotechnol 172:3810–3826. https://doi.org/10.1007/s12010-014-0772-y
Hegazi AG, El-Houssiny AS, Fouad EA (2019) Egyptian propolis 14: potential antibacterial activity of propolis-encapsulated alginate nanoparticles against different pathogenic bacteria strains. Adv Nat Sci Nanosci Nanotechnol 10:45019. https://doi.org/10.1088/2043-6254/ab52f4
Pastor C, Sánchez-González L, Cháfer M et al (2010) Physical and antifungal properties of hydroxypropylmethylcellulose based films containing propolis as affected by moisture content. Carbohydr Polym 82:1174–1183. https://doi.org/10.1016/j.carbpol.2010.06.051
In J, Raj H, Sim H et al (2014) Electrospun propolis / polyurethane composite nano fi bers for biomedical applications. Mater Sci Eng C 44:52–57. https://doi.org/10.1016/j.msec.2014.07.062
Siripatrawan U, Vitchayakitti W (2016) Improving functional properties of chitosan fi lms as active food packaging by incorporating with propolis. Food Hydrocoll 61:695–702. https://doi.org/10.1016/j.foodhyd.2016.06.001
Sester C, Ofridam F, Lebaz N et al (2020) pH-Sensitive methacrylic acid – methyl methacrylate copolymer Eudragit L100 and dimethylaminoethyl methacrylate, butyl methacrylate, and methyl methacrylate tri-copolymer Eudragit E100 to cite this version : HAL id : hal-02342633 METHACRYLATE TRI-COPOLY. Polym Adv Technol 31:440–450. https://doi.org/10.1002/pat.4780
Ding C, Zhang M, Li G (2015) Preparation and characterization of collagen / hydroxypropyl methylcellulose (HPMC) blend film. Carbohydr Polym 119:194–201. https://doi.org/10.1016/j.carbpol.2014.11.057
Refaat H, Mady FM, Sarhan HA et al (2021) Optimization and evaluation of propolis liposomes as a promising therapeutic approach for COVID-19. Int J Pharm 592:120028. https://doi.org/10.1016/j.ijpharm.2020.120028
Grecka K, Kuś PM, Okińczyc P et al (2019) The anti-staphylococcal potential of ethanolic polish Propolis extracts. Molecules 24:1732. https://doi.org/10.3390/molecules24091732
Kujumgiev A, Tsvetkova I, Serkedjieva Y et al (1999) Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol 64:235–240. https://doi.org/10.1016/S0378-8741(98)00131-7
Inouye S, Yamaguchi H, Takizawa T (2001) Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using a modified dilution assay method. J Infect Chemother 7:251–254. https://doi.org/10.1007/s101560170022
Aldana DS, Andrade-Ochoa S, Cristóbal N, Aguilar et al (2015) Antibacterial activity of pectic-based edible films incorporated with mexican lime essential oil. Food Control 50. https://doi.org/10.1016/j.foodcont.2014.10.044
Bilginer R, Umit DO, Yildiz H (2020) Biocomposite scaffolds for 3D cell culture: Propolis enriched polyvinyl alcohol nanofibers favoring cell adhesion. J Appl Polym Sci 138:50287. https://doi.org/10.1002/app.50287
Ulag S, Ilhan E, Demirhan R et al (2021) Propolis-Based nanofiber patches to repair corneal microbial keratitis. Molecules 26:2577. https://doi.org/10.3390/molecules26092577
Bagheri M, Validi M, Gholipour A, Makvandi P, Sharifi E (2022) Chitosan nanofiber biocomposites for potential wound healing applications: antioxidant activity with synergic antibacterial effect. Bioeng Transl Med 7:10254. https://doi.org/10.1002/btm2.10254
Acknowledgements
We acknowledge the financial support by the Student Research Committee, Tabriz University of Medical Sciences, Iran (Grant number: 68299).
Author information
Authors and Affiliations
Contributions
Mahdieh Abdi: Investigation; Methodology; Formal analysis; Data curation; Writing – Original draft.Parvin Zakeri-Milani: Formal analysis; Data curation.Marjan Ghorbani: Supervision, Project administration, Funding acquisition, Conceptualization, Formal analysis, Investigation, Resources, Writing – review & editing, Visualization.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethics statement
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdi, M., Zakeri-Milani, P. & Ghorbani, M. Designing and Evaluating pH-Responsive Electrospun Eudragit® L-100/Hydroxypropyl Methyl Cellulose Composite Mats for Release of Propolis as a Novel Wound Dressing. J Polym Environ 31, 3215–3229 (2023). https://doi.org/10.1007/s10924-023-02802-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-023-02802-4