Abstract
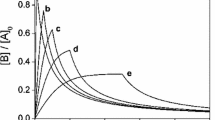
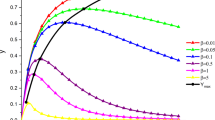
Analytical solutions are derived for a family of two-stage reactions, in which the later process is first order with respect to the product of the previous, non-first order step. A general strategy is shown that is suitable to handle typical cases. The strategy is demonstrated by considering, zeroth order, second order, mixed second order and third order initial reactions, analytical solutions for all of which can be obtained and advantageously used. The solutions can also be used as archetypes of intermediate formation and decay in chemical kinetics.




Similar content being viewed by others
References
J.H. Espenson, Chemical Kinetics and Reaction Mechanisms, 2nd edn. (McGraw-Hill, New York, 1995)
F.A. Matsen, J.L. Franklin, J. Am. Chem. Soc. 72, 3337–3341 (1950)
C.R. Quezada, T.P. Clement, K.K. Lee, Adv. Water Resour. 27, 507–520 (2004)
C. Gadgil, C.H. Lee, H.G. Othmer, Bull. Math. Biol. 67, 901–946 (2005)
W.J. Heuett, H. Qian, Bull. Math. Biol. 68, 1383–1399 (2006)
T. Jahnke, W. Huisinga, J. Math. Biol. 54, 1–26 (2007)
G. Lente, J. Chem. Phys. 137, 164101 (2012)
F. Garcia-Sevilla, M. Garcia-Moreno, M. Molina-Alarcon, M.J. Garcia-Meseguer, J.M. Villalba, E. Arribas, R. Varon, J. Math. Chem. 50, 1598–1624 (2012)
P. Érdi, G. Lente, Stochastic Chemical Kinetics (Theory and (Mostly) Systems Biological Applications (Springer, New York, 2014)
ZiTa by Gábor Peintler, http://www.staff.u-szeged.hu/~peintler/enprogs.htm
COPASI, http://www.copasi.org/
\(\text{ Pro-KIV }^{{\rm TM}}\) Kinetic Analysis & Data Simulation Software, http://www.photophysics.com/software/pro-kiv-kinetic-analysis-data-simulation-software
KINSIM/FITSIM, http://www.biochem.wustl.edu/cflab/message.html
SPECFIT Global Analysis, http://www.hi-techsci.com/products/specfitglobalanalysis/
G. Milani, F. Milani, J. Math. Chem. 50, 2577–2605 (2014)
D. Vogt, J. Math. Chem. 51, 826–842 (2013)
P. Miškinis, J. Math. Chem. 51, 1822–1834 (2013)
G. Milani, J. Math. Chem. 51, 2033–2061 (2013)
V. Vlasov, React. Kinet. Mech. Catal. 110, 5–13 (2013)
G. Milani, F. Milani, J. Math. Chem. 52, 464–488 (2014)
D. Belkić, J. Math. Chem. 52, 1201–1252 (2014)
A.A. Khadom, A.A. Abdul-Hadi, React. Kinet. Mech. Catal. 112, 15–26 (2014)
A. Izadbakhsh, A. Khatami, React. Kinet. Mech. Catal. 112, 77–100 (2014)
P. Muller, Pure Appl. Chem. 66, 1077–1184 (1994)
K.J. Laidler, Pure Appl. Chem. 68, 149–192 (1996)
Acknowledgments
The research was supported by the EU and co-financed by the European Social Fund under the project ENVIKUT (TÁMOP-4.2.2.A-11/1/KONV-2012-0043).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lente, G. Kinetics of irreversible consecutive processes with first order second steps: analytical solutions. J Math Chem 53, 1172–1183 (2015). https://doi.org/10.1007/s10910-015-0477-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10910-015-0477-7