Abstract
Cockroaches can defend themselves from threats by escape behavior. Although wind-evoked turning in Periplaneta americana is robust, little is known about the turning and translational components of the escape response to looming and localized heat stimuli, especially in other cockroach species. In particular, it has been suggested that the Madagascar hissing cockroach (Gromphadorhina portentosa) lacks an escape response. Our goal was to use high-speed video to explore both the turning and translational components of the escape response of Madagascar cockroaches to looming and heat stimuli. Our results demonstrate that, in contrast to expectations based on previous studies, Madagascar cockroaches do show an escape response that was adapted to looming direction and heat location. Although the escape response to looming stimuli was limited, the response to heat stimulation of their tarsi was unexpectedly robust, especially in translation. The translational responses to both looming and heat were directionally similar (160o and 166o). Our results demonstrate that G. portentosa also exhibits a well-organized escape response and emphasize the need to quantify both turning and translation to obtain a more complete description of an animals’ escape movement.
Similar content being viewed by others
Introduction
Insects, as well as many other invertebrates and vertebrates (reviewed in Domenici et al. 2011a, 2011b), often defend themselves from threats by escape movement (reviewed in Card 2012). Wind and looming stimuli reliably evoke escape responses in crickets (Tauber and Camhi 1995; Dupuy et al. 2011), locusts (Santer et al. 2005), grasshoppers (Cooper 2006) and cockroaches (Camhi et al. 1978). For the cockroach, most studies have focused on turning of the American cockroach (Periplaneta americana) in response to wind stimuli, showing a robust (Stierle et al. 1994) and variable (Domenici et al. 2008) response often directed nearly 180o away from the stimulus (Domenici et al. 2011b). Looming stimuli, which evoke escape responses in locust, crickets and flies, have not yet been used with cockroaches.
Although turning behavior is the most striking response of many insects to directional stimuli, translation (movement without a change in orientation) also occurs either through leaning, side-ways or forward stepping, or jumping. For example, flies lean away from looming stimuli prior to escape flight (Card and Dickinson 2008). In our laboratory, we have observed that spiders (jumping/Phidippus regius, Chilean rose/Grammostola rosea, un-published observations) translate in all directions but do not turn in response to looming and heat stimuli.
Although readily available, less is known about escape responses in cockroach species. Similar to the American cockroach, Blattela germanica (German, mentioned in McGorry et al. 2014) and the larger Blaberus discoidalis, (discoid, mentioned in Simpson et al. 1986) escape from wind. In contrast, other large species, such as Blaberus cranifer (death’s head, Simpson et al. 1986) and Gromphadorhina portentosa (Madagascar hissing cockroach, mentioned in Olsen and Triblehorn 2014; McGorry et al. 2014) only weakly escape from wind stimuli.
In contrast to the American cockroach, little is known about the behavior and escape response of the Madagascar cockroach (Bell et al. 2007). Although escape responses can be artificially evoked by combined electrical stimulation of their antennae and cerci (Erickson et al. 2015), natural escape responses to wind appear absent (mentioned in Olsen and Triblehorn 2014; Clark and Triblehorn 2014), consistent with weak cercal (Olsen and Triblehorn 2014) and interneuronal (McGorry et al. 2014) responses to wind stimuli. The apparent lack of escape response, potentially related to their strong cuticles providing protection (Clark and Triblehorn 2014), is unfortunate because Madagascar cockroaches are large, slow moving, and flightless, making them a convenient experimental model to study escape. Further supporting their experimental value, they appear to demonstrate spatial learning (Gunnarsson 2013).
Consequent to their potential viability as experimental subjects and the limited information available on their escape responses, our goal was to explore both the turning and translational components of the escape responses of Madagascar cockroaches to looming and heat stimuli. Our results demonstrate that, in contrast to expectations based on previous studies, Madagascar cockroaches do show a directional response to aversive stimuli that was adapted to stimulus direction and location. Although the response to looming stimuli was limited, the response to heat stimulation of their tarsi was unexpectedly robust. Our results further emphasize the need to quantify both turning and translation to obtain a more complete description of an animal’s’ escape movement.
Materials and Methods
Adult Madagascar hissing cockroaches (Gromphadorhina portentosa) of either sex, 51–76 mm in length, were purchased from a commercial vendor (New York Worms, Long Island, NY). They were fed carrots and dry dog food 3 times every week, and kept in a large transparent glass tank at room (with windows) conditions (23–24 °C temperature, 30–70% relative humidity, ambient environmental light/dark cycle). Experiments were conducted in the same environment during the daytime.
In order to track movement, two small circular spots (~1.0 mm diameter; e.g. visible in Fig. 2b) were marked with a white marker on the center line of the cockroaches without anesthesia. The rostral mark was positioned between the middle legs and the caudal mark was positioned toward the back of cockroach, equally distant from the rear end of the cockroach.
Cockroaches were restrained within an acrylic tube (80 mm internal diameter) on a circular white primed canvas (Canvas Pad, Artist’s Loft, Michaels) platform (200 mm in diameter) to provide friction. The cockroach was oriented to the stimulus direction or location by manually rotating the platform. After 3 min, the acrylic tube was gently removed; if the cockroach remained stationary for two seconds (which it typically did), a trial was conducted.
Looming and Heat Stimuli
The first series of experiments tested the response of the cockroach to looming stimuli, which create both visual and wind stimuli. A ball (64 mm diameter black polystyrene, McMaster-Carr, Elmhurst, IL) was projected with a 305 mm air cylinder (Grainger, Lake Forest, IL) driven by nitrogen gas (11 PSI) at 0.6 m/s toward the cockroach at a 45° vertical angle, stopping above and 20 mm horizontally from the center of the cockroach to avoid contact with both the body and antennae. The support for the air cylinder was placed on a separate table to eliminate transmitted vibration to the cockroach. Although the air cylinder created sound, control experiments with the air cylinder pointing away from cockroach never resulted in movement.
In the second series of experiments, heat was delivered to each of the six tarsi with a 980 nm infrared laser (BWF5, B&W Tek, Wilmington, DE) focused by a condenser lens to a 2 mm circle (Fig. 1). In the event the cockroach did not respond, stimulus duration was limited to one second to prevent damage. No cockroaches showed damage or abnormal movement.
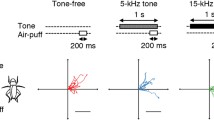
Methods. Looming and heat stimuli were delivered to Madagascar cockroaches. High speed (650 fps) video camera was placed directly over the cockroach, illuminated by an LED ring light. The cockroach was placed on a 200 mm circular white-painted canvas. Looming stimuli (80 mm black ball) were delivered at 45o. The ball stopped 20 mm from the center of the cockroach to avoid contact with its body or antennae. Stimulus (inward arrowheads) and response (outward arrowheads) directions are expressed as +180o from anterior. Heat stimulation was similar except a 980 nm focused (condenser lens) laser diode heated the tarsi. Stacked infrared filters blocked the laser light from the camera. Stimulus locations were numbered in a clockwise direction
There was no habituation with repeated trials for either looming (p = 0.06, linear mixed effects) or heat (p = 0.7, linear mixed effects) translation.
Video Recording
Video was recorded overhead with a high-speed camera (monochrome, 650 fps, 25 mm lens, f/5.8 aperture, IDT, Tallahassee, FL) controlled by propriety IDT software (Motion Studio, IDT). Illumination was provided with a low heat LED ring (Mini D-Flood, LightPanels). Each video was 1.52 s in duration and triggered at the onset of the laser or looming stimulus.
Experimental Protocol
Each cockroach was stimulated from 8 directions for the looming experiments and 6 locations for the heat experiments (Fig. 1), which were then repeated 3 times for a total of 24 or 18 trials per cockroach. Inter-stimulus interval was 4 min to minimize habituation or sensitization. The sequence of either 8 directions or 6 locations was randomized for each cockroach. Trials (30/150 for heat, 28/240 for looming) in which movement was completely absent (<0.2 mm) were not used.
Analysis
After the videos were recorded, ProAnalyst (Xcitex, Cambridge, MA) was used to track the movement of the cockroaches based on the two marked spots on the back of the cockroach. The cockroach never moved out of the field-of-view or continued to move at the end of 1.52 s. The calculated mid-point of the cockroach, which approximated its center-of-mass, was used to quantify translation (linear movement in the horizontal plane). The angle of the line between the two marked spots was used to determine the turning angle (calculated as change in angle rather than difference between final and initial angle, though in our experiments both methods yielded the same results).
Response angular change was defined as the total change in angle over the entire movement. Response translational direction and magnitude were defined as the change from beginning to the frame in which the movement exceeded a threshold of 2 mm, which separated translation from angular change and locomotion. Specifying translational angle based on a short distance minimized contamination from turning. In the event movement did not reach 2 mm (26% of trials for heat, 86% of trials for looming), direction was based on the final distance achieved. Tilting of their dorsal cuticle in response to looming stimuli had negligible effects on translation measurement. To determine if the wind associated with looming stimuli directly caused movement of the cockroach, dead cockroaches were tested (n = 2), revealing no movement (<0.01 mm). Variability in tracking prior to movement (first 5 frames), which could arise from software tracking errors or cockroach movement, was <0.06 mm (<1 pixel).
Parametric, non-parametric and circular statistics were selected based on data type and distribution and computed using Sigmaplot (Systat Software Inc., San Jose, CA), Oriana (Oriana, KCS, Wales, UK) and custom programs in Matlab (MathWorks, Natick, MA), except as described below. Effect size is represented as slope expressed as degrees (response direction) per degrees (stimulus direction or location); a slope of 1.0 represents turning or translation directly away from the stimulus. Statistical α was set to 0.05. Small p-values were capped at p < 0.00001.
Since linear mixed effect software for circular data is not commercially available, circular data (Figs. 4, 5) were analyzed with both a linear mixed effect model (random slope-intercept model, Matlab) to account for subject effects and circular (Williams-Watson, Oriana) analyses to address directional data. Similarly, both Kruskal-Wallis and a mixed linear effects model were used for response magnitude. The polar coordinate system, +180 with 0o anterior was chosen to avoid discontinuities in circular data. Similar results were always obtained.
Results
Cockroaches responded both similarly and differently to looming (10 cockroaches, 212 trials) and heat (10 cockroaches, 150 trials) stimuli. For both stimuli, cockroaches leaned away from the side of stimulation (without lifting their legs other than the one heat stimulated), followed to varying degrees by lateral and forward stepping, turning and locomotion. With looming stimuli, the cockroaches often tilted their dorsal cuticle toward the stimulus, followed by clear but limited movement (Figs. 2a, Online Resource 1). In contrast, with heat stimuli, cockroaches first lifted the affected leg, followed by leaning and significant movement (Fig. 2b, Online Resource 2). Digitized representations of the movement in Fig. 2a and b are shown in stick figures in Fig. 2c.
Typical escape responses to looming and heat stimuli. (a) Three equal interval video frames for a response to 90o looming stimulus (arrow). Registration point is indicated by the dotted lines. (b) Three equal interval video frames for a response to -135o looming stimulus (arrow). Registration point is indicated by the dotted lines. (c) Stick figures for the above responses to looming (left) and heat (right) stimuli. Filled circles represent the tracked locations. The initial position is indicated by the thicker line
The entire population of translational paths are shown in Fig. 3 for both looming (left) and heat (right) stimuli. Paths directed outward from the gray circle (which depicts the cockroach) represent movement away from the stimulated side, while paths directed inward represent movement toward the stimulus. In response to heat, all paths were directed away from the stimulus. In response to looming, paths were much shorter (note scale) with some toward the stimulus, although the largest movements were directed away from the stimulus.
Translational movement paths in response to looming (left) and heat (right) stimuli. All looming (n = 212 trials) and heat (n = 150 trials) response paths are superimposed and positioned adjacent to the indicated stimulus direction or location (see Fig. 1). Outward paths relative to the gray circle represents movement away from the stimulus, while inwards paths represent movement toward the stimulus. Note the difference in scale between the looming and heat graphs
Similarly, the entire population of angular turning trajectories are shown in Fig. 4. Again, for heat stimuli the cockroaches turned almost uniformly away from the stimulus. The turning responses to looming, however, differed from the above translational (looming, heat) and turning (heat) responses; the small responses (again, note scale) occurred both toward and away from the stimulus direction. Note also that for some trials with limited translation, rotation away from the stimulus may have resulted in the posterior portion of the cockroach actually moving closer to the stimulus.
Turning trajectories in response to looming (left) and heat (right) stimuli. All looming (n = 212) and heat (n = 150 trials) turning trajectories are superimposed and positioned adjacent to stimulus direction or location (see Fig. 1). Positive deflections indicate clockwise and negative deflections indicate counter clockwise rotation. The red lines indicated zero angular change. Negative turning trajectories for heat locations 1, 2 and 3, and looming directions 45, 90 and 135o correspond to turns away from the stimulus. Positive turning trajectories for heat locations 4, 5 and 6, and looming directions −45, −90 and -135o correspond to turns away from the stimulus. For 0o looming stimuli, either clockwise or counter clockwise corresponds to away from the stimulus. For 180o looming stimuli, any turning would be toward the stimulus. Note the difference in scale between the looming and heat graphs. Records are synchronized by the onset of the stimuli; the left and right limits of the abscissa are at 77 (50 frames) and 221 ms (~150 frames; slightly less to accommodate shorter duration responses) respectively relative to looming onset and 0 and 154 ms (100 frames) respectively relative to heat onset
The quantitative dependence of the turning responses for looming and heat stimuli are shown in Fig. 5. Consistent with the qualitative lack of dependence shown in Fig. 3, the angle of turn did not depend on the direction of looming stimulation (p = 0.1, Williams-Watson). However, turning angle did dependent significantly on the location of heat stimulation (p = 0.0001, Watson-Williams). Importantly, the shallow slope of 0.23o/o (dotted line represents a turn 180o away from the stimulus) indicates only a modest turn of 41o away from the stimulus.
Turning angle versus stimulus looming direction and heat location. (a) Escape turning angle did not depend on looming stimuli direction (p = 0.1, Williams-Watson, p = 0.1, linear mixed effects, n = 212 trials, n = 10 cockroaches). (b) Escape turning depended on heat stimulus location (p = 0.0001, Watson-Williams, p = 0.00002, linear mixed effects, n = 150 trials, slope = 0.23o/o linear regression, dotted line represents responses directed 180o away from the stimulus, n = 9 cockroaches, r2 = 0.41, Pearson correlation) on heat location. The numbers correspond to tarsi (inset, Fig. 3b)
In contrast to turning, the quantitative dependence of translational responses to both stimuli reveals a strong and more consistent dependence on stimulus location (Fig. 6). For both looming and heat stimulation, the direction of translation depended significantly (looming: p < 0.0001, heat: p < 0.00001, Williams-Watson) on stimulus direction or location. The slopes, 0.91 and 0.92o/o, indicate that the translational movements were nearly directly away (160o and 166o, respectively; dotted lines represent a turn of 180o away from the stimulus) from the stimulus.
Translation angle versus stimulus looming direction and heat location. (a) Escape translation direction depended on looming direction (p < 0.0001, Williams-Watson, p < 0.00001, linear mixed effects, slope = 0.92°/°, linear regression, n = 212 trials, n = 10 cockroaches, r2 = 0.52, Pearson correlation). Inset illustration shows stimulus and response directions. (b) Escape translation direction depended on heat stimulus location (p < 0.0001, Williams-Watson, p < 0.00001, linear mixed effects, slope = 0.91°/°, linear regression, n = 150 trials, n = 9 cockroaches, r2 = 0.91). Dotted lines represent responses directed 180o away from the stimulus. Inset illustration shows stimulus locations
The magnitude of movement, quantified as the total distance traveled before stopping, was far greater (p < 0.00001, Mann-Whitney, medians 0.22, 4.4 mm) for responses to looming rather than heat stimulation (Fig. 7). For both looming and heat stimulation, magnitude varied significantly with direction (p < 0.00001, Kruskal-Wallis) and location (p < 0.00001, Kruskal-Wallis). For looming, greater responses were obtained for stimuli directed laterally, while for heat stimulation greater responses were obtained for stimuli directed posteriorly.
Dependence of total distance traveled on looming and heat location. (a) Cockroaches translate further in response to heat than to looming stimuli (p < 0.00001, Mann-Whitney, n = 212, 150, η2 = 0.53, medians are 0.22 and 4.4 mm). (b) For looming stimuli, total distance translated varied significantly with looming direction (p < 0.00001, Kruskal-Wallis, p < 0.00001, linear mixed effects, n = 212, η2 = 0.24, medians are 0.11, 0.18, 0.36, 0.36, 0.14 mm). (c) For heat stimuli, total distance translated varied significantly with looming direction (p < 0.004, Kruskal-Wallis, p < 0.00001, linear mixed effects, n = 150, η2 = 0.06, medians are 2.8, 4.1, 9.3 mm). For all three graphs, the ordinate is logarithmic and individual data points are jittered. Box plot displays median, 25/75%, and 10/90% percentiles
Discussion
Summary
The specific aim of our research was to explore both the turning and translational components of the escape responses of Madagascar cockroaches to looming and heat stimuli. Our results demonstrate that, in contrast to expectations based on previous studies, Madagascar cockroaches do show an escape response that was robust and adapted to stimulus looming direction and heat location. Although the response to looming stimuli was limited, the response to heat stimulation of their tarsi was unexpectedly robust, especially in translation. Beyond elucidating a well-organized escape response for even small defensive movements, our results in G. portentosa emphasize the broader need to quantify both turning and translation to obtain a more complete description of an animals’ escape movement.
Cockroach Escape Responses
While previous studies have shown that the American cockroach exhibits a strong, directionally-adapted response to wind (Camhi et al. 1978), the Madagascar cockroach exhibits weak cercal responses and was believed to have little or no escape response to wind stimuli (McGorry et al. 2014; Olsen and Triblehorn 2014). Our results for looming stimuli, which contain a wind-component, support that belief, but only for response magnitude. With a median translational response of 0.22 mm (though some were > 10 mm), we suspect others might categorize the response as absent. However, although weak, the response was highly adapted to the direction of the stimulus, resulting in translation almost directly away (160o) from the stimulus. These result suggest that central neural circuits for spatial mapping of the escape response could be similar in the American and Madagascar cockroach.
Our use of localized heat stimulation of tarsi is unique. Although a common stimulus in mammalian studies (Le Bars et al. 2001), we found no previous study in which localized heat was used to evoke an escape response, making comparison to the literature difficult. Our results showed not only both turning and translational escape responses that were adapted to stimulus location, but the magnitude of response, similar to wind-evoked escape responses in the American cockroach (Camhi et al. 1978), far exceeded the responses to looming stimuli. Further, the direction of the translational response to both looming and heat were similar (160o and 166o), suggesting similar central mechanisms.
Translation Versus Turning
Insects display diverse and well-studied patterns of escape, incorporating jumping, flying, walking and turning (Card 2012). In cockroaches, the focus has been almost uniformly on turning for (for example, Camhi et al. 1978). However, flies include, through leaning (Card and Dickinson 2008), a translational component into their escape response, raising the question whether cockroaches also translate away from aversive stimuli. Our results show that although Madagascar cockroaches fail to turn, they do translate away from looming stimuli and both turn and translate away from localized heat stimuli. Translation took the form of leaning (as with flies), lateral and forward stepping. While turning and translation are not fully independent, lateral movement with or without stepping clearly differed from turning and forward locomotion. More broadly, our results in cockroaches and spiders (Cleland, unpublished results) suggest that escape translation should be more commonly evaluated in escape responses.
Mechanisms
The escape responses to looming stimuli could have been mediated by visual (Horridge 2009) and/or wind (Ye et al. 2003; Tuthill and Wilson 2016) sensory receptors. Although Madagascar cockroaches have wind sensitive cerci, they exhibit weak afferent (McGorry et al. 2014) and interneuronal (Olsen and Triblehorn 2014) responses. Vision (Okada and Toh 1998; Daltorio et al. 2013) could also have been used, however studies in the related cricket indicate that vison contributes weakly at best to the escape response (Dupuy et al. 2011). Our results showed that cockroaches escaped from stimuli directed from behind, likely out of visual range, suggesting that the cercal detection of wind was most likely responsible for evoking the escape response. If so, then the weak cercal responses (Olsen and Triblehorn 2014; McGorry et al. 2014) may explain the small magnitude of movements.
Although thermal and orienting behaviors indicate that insects (Gillott 2005), including cockroaches (Maliszewska et al. 2018), sense temperature, the location and characteristics of thermoreceptors are less clear. Regarding insect peripheral thermoreceptors, while several studies have explored thermal receptors in the antennae e.g. (Sayeed and Benzer 1996) and reviewed in (Florence and Reiser 2015), only two studies have identified heat receptors in the tarsi (Kerkut and Taylor 1957; van Haga, Henriette A Reinouts and Mitchell 1975), the anatomical target of our laser heat stimulation. In particular, heat receptors were demonstrated electrophysiologically in the American cockroach (Kerkut and Taylor 1957), suggesting similar thermoreceptors may be present in the Madagascar cockroach. Alternatively, it is possible that infrared (we used 980 nm) receptors (Robertson et al. 1996) occur on the tarsi or lower leg, though none have been reported. It is also unlikely that heat, spread to central heat receptors by diffusion through the leg, was responsible because of the length and narrowness of the leg and short latency to response.
Behavioral Implications
Our experiments were conducted in a controlled laboratory environment using non-natural stimuli. Although in response to looming stimuli cockroaches showed turning and translation adapted to stimulus orientations, the small magnitude suggests such a defense to, for example a predator, could be ineffective. Rather, tilt of the dorsal cuticle or possessing a stronger cuticle (Clark and Triblehorn 2014) may be the more useful mechanism of defense. Regarding heat stimuli, although the cockroaches showed larger turning and translational responses adapted to stimulus location, the natural source of such a heat stimulus is unclear. Ecological studies in a more natural setting are needed to explore the natural behavioral relevance of escape responses described in our report.
References
Bell WJ, Roth LM, Nalepa CA (2007) Cockroaches: ecology, behavior, and natural history. Johns Hopkins University Press, Baltimore
Camhi JM, Tom W, Volman S (1978) The escape behavior of the cockroach Periplaneta americana. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology 128:203–212
Card GM (2012) Escape behaviors in insects. Curr Opin Neurobiol 22:180–186
Card G, Dickinson MH (2008) Visually mediated motor planning in the escape response of drosophila. Curr Biol 18:1300–1307
Clark AJ, Triblehorn JD (2014) Mechanical properties of the cuticles of three cockroach species that differ in their wind-evoked escape behavior. PeerJ 2:e501
Cooper WE (2006) Risk factors and escape strategy in the grasshopper Dissosteira carolina. Behaviour 143:1201–1218
Daltorio KA, Tietz BR, Bender JA, Webster VA, Szczecinski NS, Branicky MS, Ritzmann RE, Quinn RD (2013) A model of exploration and goal-searching in the cockroach, Blaberus discoidalis. Adapt Behav 21:404–420
Domenici P, Blagburn JM, Bacon JP (2011a) Animal escapology I: theoretical issues and emerging trends in escape trajectories. J Exp Biol 214:2463–2473
Domenici P, Blagburn JM, Bacon JP (2011b) Animal escapology II: escape trajectory case studies. J Exp Biol 214:2474–2494
Domenici P, Booth D, Blagburn JM, Bacon JP (2008) Cockroaches keep predators guessing by using preferred escape trajectories. Curr Biol 18:1792–1796
Dupuy F, Casas J, Body M, Lazzari CR (2011) Danger detection and escape behaviour in wood crickets. J Insect Physiol 57:865–871
Erickson JC, Herrera M, Bustamante M, Shingiro A, Bowen T (2015) Effective stimulus parameters for directed locomotion in Madagascar hissing cockroach biobot. PLoS One 10:e0134348
Florence TJ, Reiser MB (2015) Neuroscience: hot on the trail of temperature processing. Nature 519:296
Gillott C (2005) Sensory systems. Entomology. Plenum, New York, pp 373–403
Gunnarsson KF (2013) Using Madagascar hissing cockroaches as research subjects in behavior analysis. Southern Illinois University at Carbondale
Horridge A (2009) What does an insect see? J Exp Biol 212:2721–2729
Kerkut GA, Taylor B (1957) A temperature receptor in the tarsus of the cockroach, Periplaneta americana. J Exp Biol 34:486–493
Le Bars D, Gozariu M, Cadden SW (2001) Animal models of nociception. Pharmacol Rev 53:597–652
Maliszewska J, Marciniak P, Kletkiewicz H, Wyszkowska J, Nowakowska A, Rogalska J (2018) Electromagnetic field exposure (50 Hz) impairs response to noxious heat in American cockroach. J Comp Physiol A 204:605–611
McGorry CA, Newman CN, Triblehorn JD (2014) Neural responses from the wind-sensitive interneuron population in four cockroach species. J Insect Physiol 66:59–70
Okada J, Toh Y (1998) Shade response in the escape behavior of the cockroach, Periplaneta americana. Zool Sci 15:831–835
Olsen AC, Triblehorn JD (2014) Neural responses from the filiform receptor neuron afferents of the wind-sensitive cercal system in three cockroach species. J Insect Physiol 68:76–86
Reinouts Van Haga, H., Mitchell, B. (1975) Temperature receptors on tarsi of the tsetse fly Glossina morsitans West. Nature 255, 225–226 https://doi.org/10.1038/255225a0
Robertson R, Kuhnert C, Dawson J (1996) Thermal avoidance during flight in the locust Locusta migratoria. J Exp Biol 199:1383–1393
Santer RD, Yamawaki Y, Rind FC, Simmons PJ (2005) Motor activity and trajectory control during escape jumping in the locust Locusta migratoria. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191:965–975
Sayeed O, Benzer S (1996) Behavioral genetics of thermosensation and hygrosensation in drosophila. Proc Natl Acad Sci 93:6079–6084
Simpson BS, Ritzmann RE, Pollack AJ (1986) A comparison of the escape behaviors of the cockroaches Blaberus craniifer and Periplaneta americana. J Neurobiol 17:405–419
Stierle IE, Getman M, Comer CM (1994) Multisensory control of escape in the cockroach Periplaneta americana. J Comparative Physiol A: Neuroethol Sensory, Neural, Behav Physiol 174:1–11
Tauber E, Camhi J (1995) The wind-evoked escape behavior of the cricket Gryllus bimaculatus: integration of behavioral elements. J Exp Biol 198:1895–1907
Tuthill JC, Wilson RI (2016) Mechanosensation and adaptive motor control in insects. Curr Biol 26:R1038
Van H, Reinouts HA, Mitchell BK (1975) Temperature receptors on tarsi of the tsetse fly Glossina morsitans West. Nature 255:225
Ye S, Leung V, Khan A, Baba Y, Comer CM (2003) The antennal system and cockroach evasive behavior. I Roles for visual and mechanosensory cues in the response. J Comparat Physiol A 189:89–96
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ou, J., Cleland, C.L. Escape Strategies of the Madagascar Hissing Cockroach (Gromphadorhina portentosa) in Response to Looming and Localized Heat Stimuli. J Insect Behav 32, 315–323 (2019). https://doi.org/10.1007/s10905-019-09737-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-019-09737-6