Abstract
Even though hydroxyapatite (HA) is the most common biocompatible material; it has limited antibacterial resistance. HA experiences a tailor-made structure depending on the desired applications. In this regard, silver (Ag) is of particular interest to inhibit wide spectrum of pathogenic bacteria and other microorganisms. Silver doped hydroxyapatite (Ag-HA) was developed via wet co-precipitation with subsequent hydrothermal processing to hinder the growth and multiplication of pathogenic microbes. Ag-HA demonstrated mono-dispersed nano-rods of 70 nm length and 7 nm diameters. Even though silver dopant induced stresses within the crystal lattice; Ag-HA maintained the crystallographic structure of HA with no change. Ag-HA nanocomposite demonstrated Ca/P value of 1.238 compared with 1.402 for virgin HA via XPS spectroscopy. The reduction of Ca/P value was correlated to the partial replacement of Ca+2 with Ag+1; Silver content was reported to be of 1 atomic %. Elemental mapping using EDAX confirmed uniform dispersion of silver ion within HA lattice. Antimicrobial results indicated that, Ag-HA nanocomposite demonstrated the most potent zone of inhibition (ZOI) against Staphylococcus aureus, and Candida albicans. Antibiofilm results indicated that Ag-HA nanocomposite at 10.0 µg/mL, experienced the highest percentage for S. aureus and C. albicans of 96.09%, and 77.77%, respectively. Ag-HA nanocomposite demonstrated an excellent disinfectant agent once it had excited by UV light. In growth curve assay, the OD600 value of Ag- HA were lower, showing the repression impact on the growth of S. aureus. It was observed that the quantity of cellular protein discharged from S. aureus is directly proportional to the concentration of Ag-HA, which proves the antibacterial characteristics of the Ag-HA nanocomposite, and explains the creation of holes in the bacterial membrane producing the oozing out of the proteins from the S. aureus cytoplasm. Ag-HA nanocomposite achieved the complete lysis of the bacterial cell and cell malformation, decreasing the total viable number, so the outstanding antimicrobial results encouraged the tooth filling biomedical applications.
Graphical Abstract

Similar content being viewed by others
1 Introduction
Hydroxyapatite (Ca10 (PO4)6(OH) 2; HA) constitutes 70% of human bones [1, 2]. Synthetic (HA) is the most important bioceramic material for different biomedical applications including hard tissue engineering, bone regeneration, and tissue implants [3, 4]. HA can experience excellent osteoconductive and osteoinductive properties [5]. Green HA synthesis is vital to suit the requirements for different biomedical applications [6]. HA can be developed via different techniques including sol–gel, mechanochemical, ultrasonic, combustion, and microwave processing [7]. Wet precipitation is the most potent technique; this technique could offer robust fabrication technology with controlled morphology [8]. Hydrothermal synthesis can offer highly crystallite size with controlled morphology [9]. Crystalline phase, morphology, and particle size have significant impact on HA application and its utility [10].
Nano-sized HA has better bioactivity than coarser micro-sized crystals [11, 12]. It is imperative to develop HA with consistent product quality (controlled size, surface area, and morphology) [13]. HA can exhibit various biological, mechanical, and catalytic properties [14,15,16]. HA structure could be tailored according to the desired application [17]. A considerable replacement of HA ions (including Ca+2 and PO4−) could be accomplished [18]. HA structure can tolerate a considerable number of anionic or cationic substituent that could leave the crystallographic structure unchanged [19]. HA (the most osteoconductive and biocompatible material) experienced low antibacterial resistance; bacterial infections could withstand implant failure [20]. In this regards, silver has been recognized as strong bacterial inhibitor [21]. Silver nanoparticles (NPs) have been adopted for food storage, cosmetics, textile industry, environmental applications, and bioceramic materials [22,23,24].
The integration of silver into HA matrix can offer novel antibacterial activity [25, 26]. The incorporation of silver into HA matrix has some limitations. Silver ion has larger ionic radius (1.28 Å) compared with calcium ion with smaller ionic radius (0.99 Å) [2]. Silver ion provokes stress and strain in hydroxyapatite lattice; such strains can cause stability difficulty in crystallite size [2, 27]. The theoretical limit of the Ag+ substituting Ca+2 is 20%; however the practical doping with silver ion is much lower than this limit, further details can be found in the following references [28, 29]. It is widely accepted that Ag-HA bioceramic composites can maintain the crucial physicochemical, antibacterial, and biocompatibility properties [30, 31]. Much recently, the proper integration of silver ion (Ag+) within HA structure demonstrated excellent antibacterial properties [32]. Silver was also found to be effectual to improve the lifespan of bioactive ceramics, thus overcoming the adverse effects of implant associated biofilm formation in post-surgery [33]. Silver doped hydroxyapatite can be compatible for bioceramic tooth filling with excellent antibacterial properties [34, 35]. Nasrallah D. A. and Ibrahim M. A. [36], proved that physicochemical, optical, dielectric and antimicrobial properties of polyvinyl alcohol/carboxymethyl cellulose blend films was enhanced after silver doped hydroxyapatite nanoparticles addition. Ciobanu et al., [37], synthesized silver-doped HA by co-precipitation method for studding their antibacterial and antifungal activity against pathogenic bacteria and fungi. The antimicrobial results reveals that, the synthesized nanostructure interacting differently with the microbial targets, probably due to the differences in the microbial cell wall structures. Another study conducted by Iqbal et al., [38], regarding the rapid microwave assisted synthesis silver-doped hydroxyapatite nanocomposite. The synthesized nanocomposites were conducted for disinfectant application through evaluating their antibacterial prpoperty which give an encourage results against broad spectrum pathogenic microbes.
In the present study, a facile synthesis technology including co-precipitation associated with hydrothermal processing was employed. This technology offered convenient methodology for facile development of Ag-HA nanocomposite with controlled stoichiometry. Ag-HA with consistent product quality was developed; the developed Ag-HA nanocomposite maintained the crystallite size of HA with no change. Ag-HA demonstrated nano-rods of 70 nm length and 7 nm diameters. Silver content was reported to be of 1 Atomic % using XPS. The significant impact of silver dopant on HA is the uniform purity and elevated antimicrobial, and antibiofilm activities against some microbes related to dental field which investigated to evaluate the potential impacts for biomedical, dental, and pharmaceutical applications. A review of the influence of UV light on the S. aureus (after Ag-HA treatment) was conducted case to determine whether the display time of UV light on the bacteria changed their growth. The effect of Ag-HA nanocomposite on protein leakage from bacterial cell membranes was investigated to ensure an identical reaction mechanism, so a complete and in deeps antimicrobial behavior was conducted with more discussion and related studies.
2 Materials and Methods
2.1 Chemicals
Ammonium phosphate mono-basic (NH4H2PO4) (Aldrich, 98%), Calcium nitrate tetrahydrate (Aldrich, 99%) were the precursors for HA synthesis. Ammonium hydroxide solution (Aldrich, 25%) was employed to adjust pH to 11. Silver nitrate (Aldrich, 99.99%) was employed as doping agent. All chemical were used as perceived without further treatment.
2.2 Synthesis of Ag-HA nanocomposite
Virgin HA NPs were developed via co-precipitation technique with subsequent hydrothermal processing. Calcium nitrate solution (0.2 M) were added drop wise to ammonium phosphate mono-basic (0.06 M) under magnetic stirring at 1800 rpm, and heating at 100 °C. The developed colloidal particles were transferred for batch hydrothermal processing 100 ml autoclave (Buchiglasuster, Swizerland). HA colloid was processed at 200 °C for 1 h, in an attempt to achieve highly crystallite size with balanced stoichiometric composition. Ag-HA nanocomposite was developed via partial substitution of Ca+2 with Ag+1; this was accomplished via adopting mixed solution (0.1 M AgNO3 + 0.1 M CaNO3) at the same synthesis conditions as presented in Scheme 1.
2.3 Characterization of Ag-HA Nanocomposite
Size and shape of developed Ag-HA nanocomposite were investigated using JEOL, TEM 1010 instrument (JEOL, Tokyo, Japan) with an accelerating voltage of 100 kV. Morphology of developed particles was further investigated using SEM (JWEL JSM 6010 LA, Japan) equipped with EDAX detector (X-act, Oxford instruments). The dispersion of Ag ions within HA matrix was further investigated and quantified using EDAX detector. The crystallite size of Ag-HA nanocomposite was investigated using X-ray diffraction (XRD), Siemens D-5000 powder X-ray diffractometer (Cu Ka radiation (k = 1.54056 Å). The elemental composition as well as the chemical bonding of developed Ag-HA were investigated using X-ray photoelectron spectroscopy (XPS) Thermo Scientific K-Alpha ESCA instrument equipped with aluminum Kα monochromatized radiation at 1486.6 eV X-ray source. Raman spectra were collected in powder samples deposited on glass slide as substrate, via Renishaw in Via Reflex Raman Microscope (Renishaw, Gloucestershire, UK). Experiments were conducted at room temperature using an excitation wavelength of 532 nm of 2.47 mW power, objective 20 X, and 60 s data acquisition time.
2.4 Antimicrobial Activity
Antimicrobial activity of developed Ag-HA nanocomposite to virgin HA was conducted using agar well diffusion method towards pathogenic microorganisms, including Gram-negative bacteria (Escherichia coli ATCC 25,922 & Pseudomonas aeruginosa ATCC 27,853), Gram-positive bacteria (Staphylococcus aureus ATCC 25,923 & Bacillus subtilis ATCC 6051), and unicellular fungi (Candida albicans ATCC 90,028 & Cryptococcus neoformans ATCC 14,116). The tested bacteria and unicellular fungi were inoculated on nutrient agar for one day at 37 °C. Additionally, the minimum inhibitory concentration (MIC) of HA, and Ag-HA nanocomposite was determined according to the microdilution method in agar diffusion. Different concentrations (1000–0.5 µg/mL) were performed to determine MIC. The outcomes were statistically examined using ONE WAY ANOVA, Duncan's multiple series, and the least significant difference (LSD) was determined by specific software (SPSS version 15).
2.5 Antibiofilm Potential
Furthermore, a qualitative analysis concerning biofilm restraint was described as declared by Christensen et al. [39]. The biofilm's analysis was presented at the tube wall in the absence and presence of HA, and Ag-HA nanocomposite. The antibiofilm of the as-synthesized HA, and Ag-HA nanocomposite (at 10.0 µg/mL) was measured against the chosen microbes, and correlated with the control (non-treated one). Shortly, 5 mL of the nutrient broth medium was attached inside all tubes, and the tested bacteria and yeast were inoculated, subsequent adjusted 0.5 McFarland to be 1–3.5 × 108 CFU/mL. Later that, they were incubated at 37.0 ± 0.5 °C for 24 h.
The media founded in control and treated tubes were dropped, combined with Phosphate Buffer Saline (PBS; pH 7.0), and ultimately preserved. Next, the bacterial and yeast cells that adhered to the tube walls were implanted with 5 mL sodium acetate (3.5%) for approximately 20 min. Finally, they were cleaned with deionized water. Biofilms organized inside tubes were stained with 20 mL Crystal Violet (CV; 0.15%) and washed with deionized water to eliminate the CV. It must be remarked that, for the semi-quantitative antibiofilm calculation, 5 mL of the absolute ethanol was injected to separate the stained bacterial and yeast biofilms [40]. UV–Vis. spectrophotometer at 570.0 nm had measured the O.D. of the stained bacterial and yeast biofilms [41]. The bacterial and yeast biofilms hindrance percentage was determined by using Eq. 1 [42]:
2.6 Growth Curve Assay
The influence of HA, Ag-HA nanocomposite on the growth of S. aureus (the most sensitive microbes) had been determined by the growth curve assay according to Huang et al., [43]. The bacterial suspension was adjusted to 0.5 McFarland (1 × 108 CFU/mL) in 5.0 mL of nutrient broth tubes. HA, Ag-HA nanocomposite were included separately to each examined tube. The absorbance of the bacterial growth following treatment had been evaluated, two hour time interval, for up to 24 h. (Wavelength of 600 nm) [44]. The average of duplicate readings was considered against the hour periods to get the regular growth curve.
2.7 Effect of UV-Irradiation on the Antimicrobial Activity
The examined microbes were inoculated in a nutrient broth at 37 °C for 24 h. About 0.5 mL of the incubated microbial culture were injected into 5 mL nutrient broth tubes that set following 2 h. of standard 0.5 McFarland concentration (1.5 × 108 CFU/mL of bacteria and 1 × 107 CFU/mL of Candida sp.,) [45]. After that, 100 µL Ag-HA nanocomposite were combined with the tubes and next incubated for 60 min at 36.5˚C. Tubes without Ag-HA nanocomposite, and inoculated with bacteria and unicellular yeast, and were adopted as a positive control which also displayed to UV-illumination; tubes without UV-illumination were applied as a negative control, tubes with Ag-HA nanocomposite, and tubes with Ag-HA nanocomposite and UV-illumination. Standley, a 10-W low-pressure mercury lamp, was horizontally located and used as the UV-irradiation origin, where 90% of the transmitted irradiation was at the particular wavelength. Lastly, test tubes were directed to UV-irradiation for 1 h. at a length of approximately 60 cm. After the incubation, the turbidity of the culture media was assessed at λmax of 630 nm for the unicellular fungi, and 600 nm for the tested bacteria.
2.8 Effect of Ag-HA Nanocomposite on Protein Leakage From Bacterial Cell Membranes
Pure 18 h bacterial culture was set at 0.5 McFarland (1 × 108 CFU/mL) and (100 µL) was injected into 10 mL of the nutrient broth include silver-loaded HA nanocomposite at different concentrations. Ag-HA nanocomposite-free broth injected with culture had been used as the control. All the treated samples were incubated at 37 °C for 5 h and then centrifuged for 15 min at 5000 rpm [46]. For the different samples, 100 μL supernatant had mixed with 1 mL of Bradford reagent. Optical density had measured at 595 nm after 10 min of dark incubation [46].
2.9 Reaction Mechanism Using SEM Analysis
The sensitive microbial cells (from the antibiofilm analysis) were washed with PBS three times and eventually fixed with 4.0% glutaraldehyde solution [47]. The preserved microbial cells were regularly cleaned with PBS and repeatedly drained with various ethanol concentrations (30, 50, 70, 90, and 100%) for 15 min at 28 ± 2 oC [48]. Next to that, the fixed samples were solidified on an aluminum portion, considering SEM analysis. The morphological characteristics of the control (non-treated microbial cell), and Ag-HA nanocomposite-treated microbes were observed by SEM examination.
3 Results and Discussions
3.1 Characterization of Ag-HA Nanocomposite
While HA demonstrated mono-dispersed particles of 20 nm average particle size (Fig. 1 a, b). Ag-HA nanocomposite demonstrated high quality mono-dispersed nano-rods of 70 nm length and 7 nm diameter (Fig. 1 c, d). TEM micrographs confirmed the significant impact of silver dopant on HA morphology. It can be concluded that high quality mono-dispersed Ag-HA nanocomposite was developed. The average particle size distribution of Ag-HA nanocomposite was further investigated with DLS to virgin HA (Fig. 2).
The particle size distribution via DLS was found to be in good agreement with TEM micrographs. It is apparent that silver ions were effectively integrated within the crystallite size of HA. The crystallite size of the synthesized Ag-HA nanocomposite was investigated by XRD analysis to confirm its crystallite size to virgin HA. Virgin HA demonstrated highly crystallite size with nine characteristic peaks with 2Ɵ diffraction angels at 25.8o, 31.7o, 32.2o, 32.9o, 34.0o, 39.8o, 46.7o, 49.5o, and 53.2o these peaks corresponding to the (002), (211), (112), (300), (202), (130), (222), (213), and (004) planes, respectively. These characteristic peaks were found in good accordance with ICCD card no. 00–024-0033. Ag-HA maintained the crystallite size of HA with no change (Fig. 3).
The absence of secondary crystalline phases indicated the successful partial substitution of calcium ions with silver ions without disturbing HA crystalline lattice. The average crystallite size of the synthesized Ag-HA was evaluated to virgin HA using Scherrer Equation 2.
where: D, λ, β, θ, and K are the average crystallite size, the x-ray wavelength, the width of the x-ray peak, the Bragg angle, and the so-called Scherrer constant, respectively.
It is obvious that the integration of Ag into HA matrix offered highly crystallite size, and the structure integrity of HA was maintained with no change.
Surface morphology of the synthesized Ag-HA nanocomposite were investigated using SEM, which appears as a compact particle (HA) successfully-loaded with a bright particle (Ag) as noted in Fig. 4. Elemental mapping using SEM/EDAX detector demonstrated high quality Ag-HA nanocomposite with uniform silver ion (pink color) dispersion within HA matrix (Fig. 4). Additionally, the elemental composition of the synthesized Ag-HA nanocomposite was investigated using EDAX analysis; the quantity of silver within HA was quantified as 1. 56 Atomic % as shown in Fig. 5.
XPS spectroscopy can provide vital information about chemical composition; it can be employed to identify and quantify the elemental composition of the sample. Accurate elemental binding energies (BE) were determined by referencing to the adventious C1s peak at 284.8 eV. The XPS survey spectra of Ag-HA nanocomposite to virgin HA is represented in Fig. 6.
Atomic ratios were computed from peak intensity ratios and reported the Scofield atomic sensitivity factors. Survey spectra offered the elemental composition of the sample (Table 1). The employed quantification transitions were: C1s, O1s, N1s, Ca2p, P2p, P2s, and Ag3d.
The average expected value for Ca/P in HA is 1.61. The decrease in Ca/P value can be ascribed to a thin layer of carbonaceous material; this layer is usually found on the surface of most air exposed samples. Ag-HA nanocomposite sample demonstrated decrease in Ca/P ratio; this was ascribed to the partial replacement of Ca+2 with Ag+1. The atomic percent of silver was reported to be 1.0%. The expected chemical formula of Ag-HA was Ca9.56 Ag0.44 (PO4)6 (OH)2. The binding energy Ca2 p3/2 corresponds well with the binding energy assigned to CaPO4 (347.4 eV). Even though silver content is only 1 Atomic %; it shifted the binding energy of Ca to lower values (Table 2).
The binding energy p2p corresponds well with the binding energy assigned to CaPO4 (133.2 eV). Ag-HA nanocomposite sample demonstrated slightly lower binding energy value of P2p compared with reference sample (Table 3).
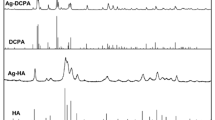
Raman spectroscopy can offer high sensitivity against secondary phase compared with XRD and FTIR. Careful examination of Raman spectra of virgin HA revealed strong absorption band at 1103 cm−1 that can be correlated to asymmetric stretch of P-O. This peak was diminished in Ag-HA sample. The symmetric stretch of P-O was shifted to low wave number at 909 cm−1 (Fig. 7).
It is widely accepted that the existence of characteristic vibration bands for PO4 3− and hydroxyl group confirmed the formation of pure hexagonal HA [2].
3.2 Antimicrobial Activity of the Synthesized HA, and Ag-HA Nanocomposite
Diffusion method was performed to evaluate the qualitative antimicrobial potential of HA, and Ag-HA nanocomposite and the results were compared with Amoxicillin/clavulanic acid and Nystatin (positive control) as presented in Fig. 8, and tabulated in Table 4.
The results revealed that Ag-HA nanocomposite render antimicrobial action toward the established pathogenic bacteria, and unicellular fungi. Additionally, the control silver-free HA possessed no antimicrobial potential. Therefore, Ag-HA nanocomposite (10 µg/mL) showed the most potent zone of inhibition (ZOI) against the bacterial strain S. aureus (15.5 mm), and unicellular fungal strain C. albicans (12.8 mm). The size of nanocomposites is not the unique parameter measuring its antimicrobial properties; further important characteristics like the elemental composition, the complete purity, surface area, and the actual shape need attention [36, 49]. Ag-HA nanocomposite is possessed advantages like the large surface-to-volume area and the nano-scale that makes it easy for it to join the biological kinds like bacteria and yeast. This is the major differences between Ag-HA nanocomposite and the general synthetic antimicrobial agents which will decrease the chance for developing antimicrobial resistance [36, 50].
The MIC results of Ag-HA nanocomposites, against all the tested microbes are in the range from 2.5 μg/mL to 0.156 μg/mL as are presented in Table 4. Ag-HA nanocomposites owns noticeable MIC as 0.165 μg/mL, and 0.312 μg/mL against S. aureus and E. coli, respectively. Additionally, a MIC result of Ag-HA nanocomposites is recorded as 1.25 μg/mL against C. albicans (see Table 4).
3.3 Antibiofilm Potential of Ag-HA Nanocomposite
The development of biofilm in pathogenic bacteria is identified by exo-polysaccharide production [41]. The tube design is used to define the antibiofilm behavior of Ag-HA nanocomposite toward some pathogenic bacteria and unicellular fungi [51].
The complete steps are: (I) Regular microbial growth and reproduction of the distinguished ring in the absence of Ag-HA nanocomposite and the inhibition of the microbial growth in the presence of Ag-HA nanocomposite, (II) The staining possibility of the established biofilm with Crystal Violet (CV), which is a semi-qualitative measurement system, and (III) Removal and separating the adhered microbial cells following ethanol reaction for the semi-quantitative evaluation of the biofilm inhibition %.
Tube methods shows the tube design to determine the antibiofilm potential of Ag-HA nanocomposite against pathogenic bacteria and Candida species, which creates a thick whitish-yellow layer in the air–liquid interface in the absence of the synthesized Ag-HA nanocomposite (control). The produced matt layers are fully adhered across the walls of the designed tubes and developed as a blue color following the staining with CV. Next, a dark blue color is created in the produced solution subsequent dissolving CV with absolute ethanol.
On the other side, in the tubes including bacterial cells and Ag-HA nanocomposite (10 µg/mL), a remarkable negative impact is noted as the cells of the tested bacteria does not produce biofilm layers, the ring construction is blocked. Also, the adherent cell color is soft, and the blue color is faintly developed following ethanol addition.
A UV–Vis. spectrophotometer is examined the semi-quantitative measurement of the inhibition %. The optical density (O.D.) was measured at 570 nm following dissolving CV-stained biofilms, which is recognized as a power of their production [51].
Table 5 illustrates the inhibition % after the addition of 10.0 µg/mL Ag-HA nanocomposite, showing that the highest percentage for S. aureus is 96.09%, for E. coli is 95.60%, and for C. albicans is 77.77%. Note that Ag-HA nanocomposite is capable of managing the biofilm extension at its adhesion strength, which is the initial start in the antimicrobial means [52].
The difference in the inhibition % is linked to several constituents like the great potential of the antimicrobial factors to be connected to the surface due to the enhanced surface area of Ag-HA nanocomposite and their particle size, as well as the attack mode and various chemical properties affecting the association and interaction of Ag-HA nanocomposite among biofilms-producing bacteria [51, 53, 54]. Figure 9 presents a review diagram concerning the antibiofilm activity of Ag-HA nanocomposite (as inhibition %) toward various pathogenic microbes.
Abdel Maksoud et al., [55], confers the reduction percentage (%) of the biofilms produced by the tested microbial strains. The most powerful restraint percentage (%) was recognized toward P. aeruginosa (98.59%) followed by C. tropicalis (94.99%) and S. aureus (93.87%) after the treatment with 10.0 μg/mL of Mn0.5Zn0.25Mg0.25Fe2O4 NPs.
3.4 Growth Curve Assay
The influence of HA, and Ag-HA nanocomposite on the growth of S. aureus had presented in Fig. 10. The growth rate of S. aureus in the control sample happened quickly, with the most potent optical density at λ = 600 nm (OD600) value and arrived at 3.15 nm. After addition of HA, no changes had been detected and OD600 was calculated to be 3.18 nm. Indifference, the OD600 value of silver-loaded HA nanocomposite were lower (0.295 nm), showing the repression impact on the growth of S. aureus. Ag-HA nanocomposite display further suppressing power rather than HA that may be defined by the unique antibacterial activity of Ag NPs that described by various previous studies [22, 44, 56, 57].
Generally, on the Ag NPs surface, the photo-generation of reactive oxygen species (ROS) has been described by early papers [36, 58, 59]. Ag-HA nanocomposite form ROS causes protein oxidation, DNA injury, and lipid peroxidation that can destroy the bacteria without harming the other cells [60]. Moreover, S. aureus membrane possesses a negative charge, while the metal ion liberated from Ag-HA nanocomposite (Ag+) own a positive charge. So, they become in direct contact point to cut to the DNA replication, protein denaturation, and bacterial cells destruction [61].
The higher hypersensitivity of the Gram-positive bacteria to the Ag NPs may be defined as a consequence of the lower stiffness of the bacterial cell membrane [62]. A further possible cause can be the size, appearance, and surface charge of Ag-HA nanocomposite, which could afford them more beneficial to connect with Gram-positive bacteria. Xu et al. [63], noted that NPs, after UV-irradiation for 80 min, cracked the cell membrane of E. coli, indicating that disinfection was performed. Most NPs, showed antibacterial potential toward different bacterial strains, like S. aureus, P. aeruginosa, and E. coli, and against broad range of pathogenic bacteria and unicellular fungi [55, 64,65,66,67,68,69,70,71].
3.5 Effect of UV-rays on the Antimicrobial potential of Ag-HA Nanocomposite
The results described in Fig. 11 demonstrates that S. aureus is more susceptible to UV light, raising its sensitivity during the exposure time. The display time of 0 to 90 min with 15 min time intervals affects the growth of S. aureus. The development of S. aureus was strongly caused by the treatment of Ag-HA nanocomposite in comparison with the untreated control sample.
In UV display, the bacterial growth ended the lowest form due to the activation by UV illumination. Because UV presentation was chosen to increase the potential for photo-activation of silver-loaded HA nanocomposite, and the possibility of silver-loaded HA nanocomposite was the highest. A consideration of the influence of UV light on the S. aureus used in this study was performed in the second case to identify whether the display time of UV light on the bacteria changed their growth. Silver-loaded HA nanocomposite is an excellent disinfectant once it had excited by UV light. In water and air requirements, Ag NPs receive photons, stopping in the construction of active hydroxyl (.OH) and new ROS (O2−, and H2O2) in the presence of O2 and H2O [72]. The ROS produced, particularly H2O2, can pass the cell membranes and produce active oxidative hydroxyl inside a reaction within bacterial cells that ultimately leads to the bacterial disinfection [58, 59].
3.6 Determination of Protein Leakage From Bacterial Cell Membranes
The quantities of protein discharged in the suspension of the treated S. aureus cells were determined applying the Bradford method [73]. From Fig. 12, it was observed that the quantity of cellular protein discharged from S. aureus is directly proportional to the concentration of silver-loaded HA nanocomposite (at different concentration) and found to be 210.63 µg/mL after the treatment with silver-loaded HA nanocomposite (1 mg/mL), which proves the antibacterial characteristics of the silver-loaded AH nanocomposite, and explains the creation of holes in the cell membrane of S. aureus producing in the oozing out of the proteins from the S. aureus cytoplasm. These test outcomes revealed that silver-loaded HA nanocomposite improved the permeability of S. aureus cell membranes more than HA. So it could be assumed that confusion of membranous permeability would be a vital portion of the repression of bacterial mass. Related studies [74] and [75] describe comparable outcomes when incorporated NPs which revealed concentration-reliant destabilization in the cell membrane of bacterial cells and point to leakage of their intracellular substance into the extracellular form (bacterial cell suspension). Paul, et al. [76] proved that the difference in bacterial cell membrane permeability was shown in percentage difference in corresponding electric conductivity. It was reported that the percentage of relative electric conductivities of all tested samples improves with the rise in the concentration of the treated nanocomposites. The integrity of the bacterial cell membrane had defined by analysis of the discharge of cell components of the bacteria such as proteins; the leakage developed with time as there was constant cell membrane injury that pointed to the leakage of cell components driving to cell destruction.
3.7 Reaction Mechanism Determination by SEM Analysis
SEM analysis had been directed to demonstrate the possible antimicrobial mechanism against S. aureus, as noted in Fig. S1. The SEM study about the control bacterial cells in the lack of silver-loaded HA nanocomposite presented bacterial groups typically prolonged with the whole regular surface, as displayed in Fig. S1 a. Following silver-loaded HA nanocomposite treatment, unusual morphological irregularities were identified in S. aureus (Fig. S1 b), including the semi-lysis of the outer surface in some bacterial cells established by deformations of the S. aureus cells. Silver-loaded HA nanocomposite performed the complete lysis of the bacterial cell and cell malformation, decreasing the total viable number (Fig. S1 b), and create holes on the surface of bacterial cells, which explains the membrane leakage assay and is confirmed by the results obtained.
The schematic illustration in Fig. 13 shows the potential antimicrobial mechanism. There were terrific superior mechanisms like Reactive Oxygen Species (ROS) distribution (superoxide anion; O2−) [21], the succession of Ag-HA nanocomposite inside the pathogenic microbes, and an alkaline tendency was admitted to show the antimicrobial action mechanism. It is recommended; metal NPs could change the microbial morphology and their biofilm formation, reduce the microbial membrane permeability and provide the residence of oxidative stress genes concerning their responses because of the H2O2 generation [21, 77]. We understand that Ag-HA nanocomposite begin their performance by wrapping and adhesion at the exterior surface of the microbial cell, producing membrane destruction and changed transport potential [55]. Then, the distribution of metal NPs (Ag NPs) inside the microbial cell divides all intracellular constructions like plasmid, DNA, and another essential organelle. Ultimately, cellular toxicity happens due to the oxidative stress created by the generation of ROS. Lastly, at acidic medium, the ionic species were created (Ag+ ions), which making cellular and genotoxicity due to the interaction among the negatively charged vital organs [55, 65].
Schematic description about the four main steps of antibacterial action of Ag-HA nanocomposite, where (1)—Ag-HA nanocomposite on the bacterial cell outside and finishes in membrane destruction, followed by endocytosis and endosome formation and changed transport potential. (2)—Ag-HA nanocomposite hinder the ions transportation from and over the bacterial cell. (3)—Ag-HA nanocomposite generate and enhance the ROS pointing to bacterial cell wall weakening. (4)—Ag-HA nanocomposite enter the bacterial cells and combine with cellular organelles, influencing respective cellular devices, modulating the cellular signal operation, and beginning cell destruction. Ag-HA nanocomposite may assist as a carrier to efficiently release Ag+ ions to the cytoplasm and layer, where proton motive power would lower the pH to be more invisible than 3.5 and promote the discharge of Ag+ ions
4 Conclusion
In the present study, a facile synthesis technology including co-precipitation associated with hydrothermal processing was employed. This technology offered convenient methodology for facile development of Ag-HA nanocomposite with controlled stoichiometry. The synthesized Ag-HA nanocomposite demonstrated mono-dispersed nano-rods of 70 nm length and 7 nm diameters. Ag-HA leaves the crystallographic structure of HA unchanged. Practical value of Ca/P for Ag/HA was reported to be 1.238 compared with 1.402 for virgin HA. The decrease of Ca/P value was correlated to the partial replacement of Ca+2 with silver ion. Silver content was reported to be of 1 Atomic % using XPS. Elemental mapping using EDS confirmed uniform dispersion of silver ion within the HA lattice. Antimicrobial results revealed that Ag-HA nanocomposite (10 µg/mL) exhibited the most potent zone of inhibition (ZOI) against S. aureus (15.5 mm) and C. albicans (12.8 mm). The size of Ag-HA nanocomposite is not the unique parameter measuring its antimicrobial properties; further important characteristics like the elemental composition, the complete purity, surface area, loaded-Ag NPs activity, and the actual shape need attention. Ag-HA nanocomposites owns novel MIC as 0.165 μg/mL, and 0.312 μg/mL against S. aureus and E. coli, respectively. Additionally, MIC result of Ag-HA nanocomposites is recorded as 1.25 μg/mL against C. albicans. Antibiofilm results demonstrate that, after adding 10.0 µg/mL Ag-HA nanocomposite, the highest inhibition percentage for S. aureus is 96.09%, for E. coli is 95.60%, and for C. albicans is 77.77%. The growth rate of S. aureus in the control sample happened quickly, with the most potent optical density at λ = 600 nm (OD600) value arrived at 3.15 nm. After addition of HA, no changes gad been detected and OD600 was calculated to be 3.18 nm. Indifference, the OD600 value of silver-loaded HA nanocomposite were lower (0.295 nm), showing the repression impact on the growth of S. aureus. Ag-HA nanocomposite achieved the complete lysis of the bacterial cell and cell malformation, decreasing the total viable number and creating holes on the surface of bacterial cells, which explains the membrane leakage assay and is approved by the results obtained for encouraged the tooth filling biomedical applications.
References
M.F. Mohd Yusoff, N.H. Abu Kasim, W.H. Himratul-Aznita, S. Saidin, K. Genasan, T. Kamarul, Z. Radzi, Physicochemical, antibacterial and biocompatibility assessments of silver incorporated nano-hydroxyapatite synthesized using a novel microwave-assisted wet precipitation technique. Mater. Charact. (2021). https://doi.org/10.1016/j.matchar.2021.111169
M. Riaz, R. Zia, A. Ijaz, T. Hussain, M. Mohsin, A. Malik, Synthesis of monophasic Ag doped hydroxyapatite and evaluation of antibacterial activity. Mater. Sci. Eng. C. 90, 308–313 (2018)
S. Elbasuney, H.E. Mostafa, Synthesis and surface modification of nanophosphorous-based flame retardant agent by continuous flow hydrothermal synthesis. Particuology 22, 82–88 (2015)
S. Elbasuney, Green synthesis of hydroxyapatite nanoparticles with controlled morphologies and surface properties toward biomedical applications. J. Inorg. Organomet. Polym. Mater. 30(3), 899–906 (2020)
R. James, M. Deng, C.T. Laurencin, S.G. Kumbar, Nanocomposites and bone regeneration. Front. Mater. Sci. 5(4), 342–357 (2011)
Z. Beyene, R. Ghosh, Effect of zinc oxide addition on antimicrobial and antibiofilm activity of hydroxyapatite: a potential nanocomposite for biomedical applications. Mater. Today Commun. 21, 100612 (2019)
S. Elbasuney, A. Maraden, Novel thermoset nanocomposite intumescent coating based on hydroxyapatite nanoplates for fireproofing of steel structures. J. Inorg. Organomet. Polym Mater. 30(3), 820–830 (2020)
J. Lv, L. Qiu, B. Qu, Controlled growth of three morphological structures of magnesium hydroxide nanoparticles by wet precipitation method. J. Cryst. Growth 267(3–4), 676–684 (2004)
B. Baruwati, D.K. Kumar, S.V. Manorama, Hydrothermal synthesis of highly crystalline ZnO nanoparticles: a competitive sensor for LPG and EtOH. Sens. Actuators, B Chem. 119(2), 676–682 (2006)
S. Bose, S.K. Saha, Synthesis and characterization of hydroxyapatite nanopowders by emulsion technique. Chem. Mater. 15(23), 4464–4469 (2003)
H.-J. Song, J.-W. Kim, M.-S. Kook, W.-J. Moon, Y.-J. Park, Fabrication of hydroxyapatite and TiO2 nanorods on microarc-oxidized titanium surface using hydrothermal treatment. Appl. Surf. Sci. 256(23), 7056–7061 (2010)
L.-P. Yan, J. Silva-Correia, C. Correia, S.G. Caridade, E.M. Fernandes, R.A. Sousa, J.F. Mano, J.M. Oliveira, A.L. Oliveira, R.L. Reis, Bioactive macro/micro porous silk fibroin/nano-sized calcium phosphate scaffolds with potential for bone-tissue-engineering applications. Nanomedicine 8(3), 359–378 (2013)
E. Bouyer, F. Gitzhofer, M. Boulos, Morphological study of hydroxyapatite nanocrystal suspension. J. Mater. Sci.—Mater. Med. 11(8), 523–531 (2000)
S. Park, J. Choi, S. Mondal, T.M.T. Vo, H. Lee, S.Y. Nam, C.-S. Kim, J. Oh, The impact of Cu (II) ions doping in nanostructured hydroxyapatite powder: a finite element modelling study for physico-mechanical and biological property evaluation. Adv. Powder Technol. (2021). https://doi.org/10.1016/j.apt.2021.103405
B. Nayak, A. Samant, P.K. Misra, M. Saxena, Nanocrystalline hydroxyapatite: a potent material for adsorption, biological and catalytic studies. Mater. Today 9, 689–698 (2019)
R.A. Youness, M.A. Taha, M.A. Ibrahim, Effect of sintering temperatures on the in vitro bioactivity, molecular structure and mechanical properties of titanium/carbonated hydroxyapatite nanobiocomposites. J. Mol. Struct. 1150, 188–195 (2017)
H. Donya, R. Darwesh, M. Ahmed, Morphological features and mechanical properties of nanofibers scaffolds of polylactic acid modified with hydroxyapatite/CdSe for wound healing applications. Int. J. Biol. Macromol. 186, 897–908 (2021)
Z. Li, W. Lam, C. Yang, B. Xu, G. Ni, S. Abbah, K. Cheung, K. Luk, W. Lu, Chemical composition, crystal size and lattice structural changes after incorporation of strontium into biomimetic apatite. Biomaterials 28(7), 1452–1460 (2007)
D. Laurencin, N. Almora-Barrios, N.H. de Leeuw, C. Gervais, C. Bonhomme, F. Mauri, W. Chrzanowski, J.C. Knowles, R.J. Newport, A. Wong, Magnesium incorporation into hydroxyapatite. Biomaterials 32(7), 1826–1837 (2011)
E. Yılmaz, F. Çalışkan, A new functional graded dental implant design with biocompatible and antibacterial properties. Mater. Chem. Phys. 277, 125481 (2022)
A.I. El-Batal, F.M. Mosallam, G.S. El-Sayyad, Synthesis of metallic silver nanoparticles by fluconazole drug and gamma rays to inhibit the growth of multidrug-resistant microbes. J. Cluster Sci. 29(6), 1003–1015 (2018)
A. Baraka, S. Dickson, M. Gobara, G.S. El-Sayyad, M. Zorainy, M.I. Awaad, H. Hatem, M.M. Kotb, A. Tawfic, Synthesis of silver nanoparticles using natural pigments extracted from Alfalfa leaves and its use for antimicrobial activity. Chem. Pap. 71(11), 2271–2281 (2017)
M.M. Ghobashy, M. Abd Elkodous, S.H. Shabaka, S.A. Younis, D.M. Alshangiti, M. Madani, S.A. Al-Gahtany, W.F. Elkhatib, A.M. Noreddin, N. Nady, An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment. Nanotechnol. Rev. 10(1), 954–977 (2021)
A.-C. Burdușel, O. Gherasim, A.M. Grumezescu, L. Mogoantă, A. Ficai, E. Andronescu, Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials 8(9), 681 (2018)
A.A. Hassan, H.A. Radwan, S.A. Abdelaal, N.S. Al-Radadi, M. Ahmed, K.R. Shoueir, M.A. Hady, Polycaprolactone based electrospun matrices loaded with Ag/hydroxyapatite as wound dressings: Morphology, cell adhesion, and antibacterial activity. Int. J. Pharm. 593, 120143 (2021)
O. Braissant, P. Chavanne, M. de Wild, U. Pieles, S. Stevanovic, R. Schumacher, L. Straumann, D. Wirz, P. Gruner, A. Bachmann, Novel microcalorimetric assay for antibacterial activity of implant coatings: The cases of silver-doped hydroxyapatite and calcium hydroxide. J. Biomed. Mater. Res. B Appl. Biomater. 103(6), 1161–1167 (2015)
K.E. Mosaad, K.R. Shoueir, M.M. Dewidar, Fabrication of multifunctional wound dressing composite biomaterials composed of Ag/Mg-Hydroxyapatite doped electrospun poly (Vinyl Alcohol) nanofibers for skin tissue regeneration. J. Clust. Sci. (2021). https://doi.org/10.1007/s10876-021-02195-1
M. Sayahi, J. Santos, H. El-Feki, C. Charvillat, F. Bosc, I. Karacan, B. Milthorpe, C. Drouet, Brushite (Ca, M) HPO4, 2H2O doping with bioactive ions (M= Mg2+, Sr2+, Zn2+, Cu2+, and Ag+): a new path to functional biomaterials? Mater. Today Chem. 16, 100230 (2020)
Y. Huang, G. Song, X. Chang, Z. Wang, X. Zhang, S. Han, Z. Su, H. Yang, D. Yang, X. Zhang, Nanostructured Ag+-substituted fluorhydroxyapatite-TiO2 coatings for enhanced bactericidal effects and osteoinductivity of Ti for biomedical applications. Int. J. Nanomed. 13, 2665 (2018)
M.F.M. Yusoff, N.H.A. Kasim, W.H. Himratul-Aznita, S. Saidin, K. Genasan, T. Kamarul, Z. Radzi, Physicochemical, antibacterial and biocompatibility assessments of silver incorporated nano-hydroxyapatite synthesized using a novel microwave-assisted wet precipitation technique. Mater. Charact. 178, 111169 (2021)
K. Szurkowska, A. Laskus, J. Kolmas, Hydroxyapatite-based materials for potential use in bone tissue infections, Hydroxyapatite—Advances in Composite Nanomaterials, Biomedical Applications and Its Technological Facets (London. UK, IntechOpen, 2018), pp.109–135
B. Wang, L. Ma, L. Xie, H. Yang, Y. Li, S. Wang, H. Qiao, H. Lin, J. Lan, Y. Huang, Chemical stability, antibacterial and osteogenic activities study of strontium-silver co-substituted fluorohydroxyapatite nanopillars: A potential multifunctional biological coating. Ceram. Int. 46(17), 27758–27773 (2020)
A. Simchi, E. Tamjid, F. Pishbin, A. Boccaccini, Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications, nanomedicine: nanotechnology. Biol. Med. 7(1), 22–39 (2011)
M.H. Malekipour Esfahani, N. Sharifinezhad, M. Hemati, A.M. Gholami, Evaluation of mechanical properties of bioglass materials for dentistry application. J. Simul. Anal. Novel Technol. Mech. Eng. 13(4), 19–29 (2021)
G. Nedunchezhian, D.B. Anburaj, B. Gokulakumar, S.J. Jeyakumar, Microwave assisted synthesis and charecterization of silver and zinc doped hydroxyapatite nanorods from mussel shell (MOLLUSK). Rom J. Biophys. 26(1), 11–20 (2016)
D.A. Nasrallah, M.A. Ibrahim, Enhancement of physico-chemical, optical, dielectric and antimicrobial properties of polyvinyl alcohol/carboxymethyl cellulose blend films by addition of silver doped hydroxyapatite nanoparticles. J. Polym. Res. 29(3), 86 (2022)
C.S. Ciobanu, S.L. Iconaru, M.C. Chifiriuc, A. Costescu, P. Le Coustumer, D. Predoi, Synthesis and antimicrobial activity of silver-doped hydroxyapatite nanoparticles. BioMed. Res. Int. (2013). https://doi.org/10.1155/2013/916218
N. Iqbal, M.R.A. Kadir, N.A.N.N. Malek, N.H. Mahmood, M.R. Murali, T. Kamarul, Rapid microwave assisted synthesis and characterization of nanosized silver-doped hydroxyapatite with antibacterial properties. Mater. Lett. 89, 118–122 (2012)
G.D. Christensen, W.A. Simpson, A.L. Bisno, E.H. Beachey, Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 37(1), 318–326 (1982)
N. Narisawa, S. Furukawa, H. Ogihara, M. Yamasaki, Estimation of the biofilm formation of E. coli K-12 by the cell number. J. Biosci. Bioeng. 99(1), 78–80 (2005)
A.I. El-Batal, G.S. El-Sayyad, N.E. Al-Hazmi, M. Gobara, Antibiofilm and antimicrobial activities of silver boron nanoparticles synthesized by pvp polymer and gamma rays against urinary tract pathogens. J. Cluster Sci. 30(4), 947–964 (2019)
M.A. Ansari, H.M. Khan, A.A. Khan, S.S. Cameotra, R. Pal, Antibiofilm efficacy of silver nanoparticles against biofilm of extended spectrum β-lactamase isolates of E. coli and Klebsiella pneumoniae. Appl. Nanosci. 4(7), 859–868 (2014)
W. Huang, J.-Q. Wang, H.-Y. Song, Q. Zhang, G.-F. Liu, Chemical analysis and in vitro antimicrobial effects and mechanism of action of Trachyspermum copticum essential oil against E. coli. Asian. Pac. J. Trop. Med. 10(7), 663–669 (2017)
M. Bekhit, S.H. El-Sabbagh, R.M. Mohamed, G.S. El-Sayyad, R. Sokary, Mechanical thermal and antimicrobial properties of LLDPE/EVA/MMT/Ag nanocomposites films synthesized by gamma irradiation. J. Inorg. Organomet. Polym. (2021). https://doi.org/10.1007/s10904-021-02137-4
K.F. El-Nemr, H.R. Mohamed, M.A. Ali, R.M. Fathy, A.S. Dhmees, Polyvinyl alcohol/gelatin irradiated blends filled by lignin as green filler for antimicrobial packaging materials. Int. J. Environ. Anal. Chem. 100(14), 1578–1602 (2020)
H. Agarwal, A. Nakara, S. Menon, V. Shanmugam, Eco-friendly synthesis of zinc oxide nanoparticles using Cinnamomum Tamala leaf extract and its promising effect towards the antibacterial activity. J. Drug Deliv Sci Technol 53, 101212 (2019)
A.N. El-Shazly, G.S. El-Sayyad, A.H. Hegazy, M.A. Hamza, R.M. Fathy, E. El Shenawy, N.K. Allam, Superior visible light antimicrobial performance of facet engineered cobalt doped TiO 2 mesocrystals in pathogenic bacterium and fungi. Sci. Rep. 11(1), 1–14 (2021)
A.M. El-Khawaga, A.A. Farrag, M.A. Elsayed, G.S. El-Sayyad, A.I. El-Batal, Antimicrobial and photocatalytic degradation activities of chitosan-coated magnetite nanocomposite. J. Clust. Sci. (2020). https://doi.org/10.1007/s10876-020-01869-6
M. Abd Elkodous, G.S. El-Sayyad, A.E. Mohamed, K. Pal, N. Asthana, F.G. de Souza Junior, F.M. Mosallam, M. Gobara, A.I. El-Batal, Layer-by-layer preparation and characterization of recyclable nanocomposite (Co x Ni 1− x Fe 2 O 4; X= 0.9/SiO 2/TiO 2). J. Mater. Sci. 30(9), 8312–8328 (2019)
K. Pal, A. Si, G.S. El-Sayyad, M.A. Elkodous, R. Kumar, A.I. El-Batal, S. Kralj, S. Thomas, Cutting edge development on graphene derivatives modified by liquid crystal and CdS/TiO2 hybrid matrix: optoelectronics and biotechnological aspects. Crit. Rev. Sol. State Mater. Sci. (2020). https://doi.org/10.1080/10408436.2020.1805295
A.I. El-Batal, H.G. Nada, R.R. El-Behery, M. Gobara, G.S. El-Sayyad, Nystatin-mediated bismuth oxide nano-drug synthesis using gamma rays for increasing the antimicrobial and antibiofilm activities against some pathogenic bacteria and candida species. RSC Adv. 10(16), 9274–9289 (2020)
A.F. de Faria, D.S.T. Martinez, S.M.M. Meira, A.C.M. de Moraes, A. Brandelli, A.G. Souza Filho, O.L. Alves, Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Coll. Surf. B. 113, 115–124 (2014)
F. Martinez-Gutierrez, L. Boegli, A. Agostinho, E.M. Sánchez, H. Bach, F. Ruiz, G. James, Anti-biofilm activity of silver nanoparticles against different microorganisms. Biofouling 29(6), 651–660 (2013)
P.P. Mahamuni, P.M. Patil, M.J. Dhanavade, M.V. Badiger, P.G. Shadija, A.C. Lokhande, R.A. Bohara, Synthesis and characterization of zinc oxide nanoparticles by using polyol chemistry for their antimicrobial and antibiofilm activity. Biochem. Biophys. rep. 17, 71–80 (2019)
M.A. Maksoud, G.S. El-Sayyad, A.M. El-Khawaga, M. Abd Elkodous, A. Abokhadra, M.A. Elsayed, M. Gobara, L. Soliman, H. El-Bahnasawy, A. Ashour, Nanostructured Mg substituted Mn-Zn ferrites: a magnetic recyclable catalyst for outstanding photocatalytic and antimicrobial potentials. J. Hazardous Mater. 399, 123000 (2020)
M.A. Maksoud, G.S. El-Sayyad, H.S. El-Bastawisy, R.M. Fathy, Antibacterial and antibiofilm activities of silver-decorated zinc ferrite nanoparticles synthesized by a gamma irradiation-coupled sol–gel method against some pathogenic bacteria from medical operating room surfaces. RSC Adv. 11(45), 28361–28374 (2021)
A.I. El-Batal, M. Abd Elkodous, G.S. El-Sayyad, N.E. Al-Hazmi, M. Gobara, A. Baraka, Gum arabic polymer-stabilized and gamma rays-assisted synthesis of bimetallic silver-gold nanoparticles: powerful antimicrobial and antibiofilm activities against pathogenic microbes isolated from diabetic foot patients. Int. J. Biol. Macromol. 165, 169–186 (2020)
R.M. Fathy, A.Y., Mahfouz, Eco-friendly graphene oxide-based magnesium oxide nanocomposite synthesis using fungal fermented by-products and gamma rays for outstanding antimicrobial, antioxidant, and anticancer activities. J. Nanostructure Chem 1–21, 301–321 (2021)
A. Joe, S.-H. Park, D.-J. Kim, Y.-J. Lee, K.-H. Jhee, Y. Sohn, E.-S. Jang, Antimicrobial activity of ZnO nanoplates and its Ag nanocomposites: insight into an ROS-mediated antibacterial mechanism under UV light. J. Sol. State Chem. 267, 124–133 (2018)
M.M. Naik, H.B. Naik, G. Nagaraju, M. Vinuth, H.R. Naika, K. Vinu, Green synthesis of zinc ferrite nanoparticles in Limonia acidissima juice: characterization and their application as photocatalytic and antibacterial activities. Microchem. J. 146, 1227–1235 (2019)
G. Sharmila, M. Thirumarimurugan, C. Muthukumaran, Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J. 145, 578–587 (2019)
A. Samavati, M. Mustafa, A. Ismail, M. Othman, M. Rahman, Copper-substituted cobalt ferrite nanoparticles: structural, optical and antibacterial properties. Mater. Express 6(6), 473–482 (2016)
Y. Xu, Q. Liu, M. Xie, S. Huang, M. He, L. Huang, H. Xu, H. Li, Synthesis of zinc ferrite/silver iodide composite with enhanced photocatalytic antibacterial and pollutant degradation ability. J. Coll. Interface Sci. 528, 70–81 (2018)
S. Patil, H.B. Naik, G. Nagaraju, R. Viswanath, S. Rashmi, Sugarcane juice mediated eco-friendly synthesis of visible light active zinc ferrite nanoparticles: application to degradation of mixed dyes and antibacterial activities. Mater. Chem. Phys. 212, 351–362 (2018)
A. Ashour, A.I. El-Batal, M.A. Maksoud, G.S. El-Sayyad, S. Labib, E. Abdeltwab, M. El-Okr, Antimicrobial activity of metal-substituted cobalt ferrite nanoparticles synthesized by sol–gel technique. Particuology 40, 141–151 (2018)
M.A. Maksoud, G.S. El-Sayyad, A. Ashour, A.I. El-Batal, M.S. Abd-Elmonem, H.A. Hendawy, E. Abdel-Khalek, S. Labib, E. Abdeltwab, M. El-Okr, Synthesis and characterization of metals-substituted cobalt ferrite [Mx Co (1-x) Fe2O4;(M= Zn, Cu and Mn; x= 0 and 0.5)] nanoparticles as antimicrobial agents and sensors for Anagrelide determination in biological samples. Mater. Sci. Eng. 92, 644–656 (2018)
M.A. Maksoud, G.S. El-Sayyad, A. Ashour, A.I. El-Batal, M.A. Elsayed, M. Gobara, A.M. El-Khawaga, E. Abdel-Khalek, M. El-Okr, Antibacterial, antibiofilm, and photocatalytic activities of metals-substituted spinel cobalt ferrite nanoparticles. Microb. Pathog. 127, 144–158 (2019)
M.A. Maksoud, G.S. El-Sayyad, A. Abokhadra, L. Soliman, H. El-Bahnasawy, A. Ashour, Influence of Mg 2+ substitution on structural, optical, magnetic, and antimicrobial properties of Mn–Zn ferrite nanoparticles. J. Mater. Sci. 31(3), 2598–2616 (2020)
G.S. El-Sayyad, M. Abd Elkodous, A.M. El-Khawaga, M.A. Elsayed, A.I. El-Batal, M. Gobara, Merits of photocatalytic and antimicrobial applications of gamma-irradiated Co x Ni 1− x Fe 2 O 4/SiO 2/TiO 2; x= 0.9 nanocomposite for pyridine removal and pathogenic bacteria/fungi disinfection: implication for wastewater treatment. RSC Adv. 10(9), 5241–5259 (2020)
M. Abd Elkodous, G.S. El-Sayyad, S.M. Youssry, H.G. Nada, M. Gobara, M.A. Elsayed, A.M. El-Khawaga, G. Kawamura, W.K. Tan, A.I. El-Batal, Carbon-dot-loaded Co x Ni 1− x Fe 2 O 4; x= 0.9/SiO 2/TiO 2 nanocomposite with enhanced photocatalytic and antimicrobial potential: An engineered nanocomposite for wastewater treatment. Sci. Rep. 10(1), 1–22 (2020)
M. Abd Elkodous, G.S. El-Sayyad, M.A. Maksoud, R. Kumar, K. Maegawa, G. Kawamura, W.K. Tan, A. Matsuda, Nanocomposite matrix conjugated with carbon nanomaterials for photocatalytic wastewater treatment. J. Hazardous Mater. 410, 124657 (2021)
L.F. Gaunt, C.B. Beggs, G.E. Georghiou, Bactericidal action of the reactive species produced by gas-discharge nonthermal plasma at atmospheric pressure: a review. IEEE Trans. Plasm. Sci. 34(4), 1257–1269 (2006)
N. Bradford, A rapid and sensitive method for the quantitation microgram quantities of a protein isolated from red cell membranes. Anal. Biochem. 72(248), e254 (1976)
S. Rajesh, V. Dharanishanthi, A.V. Kanna, Antibacterial mechanism of biogenic silver nanoparticles of Lactobacillus acidophilus. J. Exp. Nanosci. 10(15), 1143–1152 (2015)
Z. Azam, A. Ayaz, M. Younas, Z. Qureshi, B. Arshad, W. Zaman, F. Ullah, M.Q. Nasar, S. Bahadur, M.M. Irfan, Microbial synthesized cadmium oxide nanoparticles induce oxidative stress and protein leakage in bacterial cells. Microb. Pathog. 144, 104188 (2020)
D. Paul, S. Maiti, D.P. Sethi, S. Neogi, Bi-functional NiO-ZnO nanocomposite: synthesis, characterization, antibacterial and photo assisted degradation study. Adv. Powder Technol. 32(1), 131–143 (2021)
M. Abd Elkodous, G.S. El-Sayyad, I.Y. Abdelrahman, H.S. El-Bastawisy, A.E. Mohamed, F.M. Mosallam, H.A. Nasser, M. Gobara, A. Baraka, M.A. Elsayed, A.I. El-Batal, Therapeutic and diagnostic potential of nanomaterials for enhanced biomedical applications. Coll Surfaces B: Biointerfaces. 180, 411–428 (2019)
Acknowledgements
The authors would like to thank Prof. Mohamed Gobara (Military Technical College, Egyptian Armed Forces), and Zeiss microscope team in Cairo for their invaluable advice during this study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Research Involving Human and Animal Participation
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elbasuney, S., El-Sayyad, G.S., Radwan, S.M. et al. Antimicrobial, and Antibiofilm Activities of Silver Doped Hydroxyapatite: A Novel Bioceramic Material for Dental Filling. J Inorg Organomet Polym 32, 4559–4575 (2022). https://doi.org/10.1007/s10904-022-02459-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02459-x