Abstract
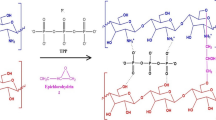
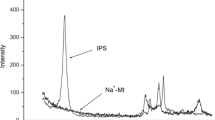
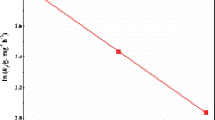
In this work, starch–montmorillonite/polyaniline (St–Mt/PANI) nanocomposite was prepared by in situ polymerization of aniline on the surface of starch–montmorillonite (St–Mt) nanocomposite and was applied for adsorption and removal of Cr(VI) ions from aqueous solution in batch mode. Various characterization techniques such as Fourier transform infrared spectroscopy, X-ray diffraction, thermogravimetric analysis, and scanning electron microscopy were utilized to characterize and evaluate the prepared nanocomposite. In order to verify the optimum conditions for adsorption of Cr(VI) ions by St–Mt nanocomposite, as powder, different parameters affecting the adsorption efficiency including pH of the solution, the initial concentration of Cr(VI) ions, contact time and adsorbent dosage were investigated. According to the results, the best conditions for Cr(VI) removal was obtained as pH 2, C0 = 10 mg L−1, time = 15 min and M = 0.05 g. The adsorption process was well fitted by Langmuir isotherm model and the maximum adsorption capacity was calculated as 208.33 mg g−1. Also, the kinetic adsorption process was well described by the pseudo-second-order kinetic model. The study of thermodynamic parameters confirmed the spontaneous and exothermic nature of adsorption and removal process. Desorption tests revealed the reusability of St–Mt/PANI nanocomposite for three adsorption cycles and so the method is almost cost efficient.









Similar content being viewed by others
References
J. Yang, M. Yu, W. Chen, Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from longan seed: kinetics, equilibrium and thermodynamics. J. Ind. Eng. Chem. 21, 414–422 (2015)
A. Maleki, B. Hayati, M. Naghizadeh, S.W. Joo, Adsorption of hexavalent chromium by metal organic frameworks from aqueous solution. J. Ind. Eng. Chem. 28, 211–216 (2015)
S.I. Rathnayake, W.N. Martens, Y. Xi, R.L. Frost, G.A. Ayoko, Remediation of Cr(VI) by inorganic-organic clay. J. Colloid Interface Sci. 490, 163–173 (2017)
S. Rengaraj, K.-H. Yeon, S.-H. Moon, Removal of chromium from water and wastewater by ion exchange resins. J. Hazard. Mater. 87, 273–287 (2001)
G. Wang, Y. Hua, X. Su, S. Komarneni, S. Ma, Y. Wang, Cr (VI) adsorption by montmorillonite nanocomposites. Appl. Clay Sci. 124, 111–118 (2016)
A. Çimen, F. Kılıçel, G. Arslan, Removal of chromium ions from waste waters using reverse osmosis AG and SWHR membranes. Russ. J. Phys. Chem. A 88, 845–850 (2014)
L. Alidokht, A. Khataee, A. Reyhanitabar, S. Oustan, Reductive removal of Cr(VI) by starch-stabilized Fe0 nanoparticles in aqueous solution. Desalination 270, 105–110 (2011)
X. Liu, W. Zhou, X. Qian, J. Shen, X. An, Polyaniline/cellulose fiber composite prepared using persulfate as oxidant for Cr(VI)-detoxification. Carbohydr. Polym. 92, 659–661 (2013)
Y. Kong, J. Wei, Z. Wang, T. Sun, C. Yao, Z. Chen, Heavy metals removal from solution by polyaniline/palygorskite composite. J. Appl. Polym. Sci. 122, 2054–2059 (2011)
R. Karthik, S. Meenakshi, Removal of hexavalent chromium ions using polyaniline/silica gel composite. J. Water Process Eng. 1, 37–45 (2014)
A. Olad, R. Nabavi, Application of polyaniline for the reduction of toxic Cr(VI) in water. J. Hazard. Mater. 147, 845–851 (2007)
B.S. Kadu, Y.D. Sathe, A.B. Ingle, R.C. Chikate, K.R. Patil, C.V. Rode, Efficiency and recycling capability of montmorillonite supported Fe–Ni bimetallic nanocomposites towards hexavalent chromium remediation. Appl. Catal. B 104, 407–414 (2011)
S.-L. Bee, M. Abdullah, S.-T. Bee, L.T. Sin, A. Rahmat, Polymer nanocomposites based on silylated-montmorillonite: a review. Prog. Polym. Sci. 85, 57–82 (2018)
L. Ai, L. Li, Efficient removal of organic dyes from aqueous solution with ecofriendly biomass-derived carbon@ montmorillonite nanocomposites by one-step hydrothermal process. Chem. Eng. J. 223, 688–695 (2013)
N. Ghaemi, S.S. Madaeni, A. Alizadeh, H. Rajabi, P. Daraei, Preparation, characterization and performance of polyethersulfone/organically modified montmorillonite nanocomposite membranes in removal of pesticides. J. Membr. Sci. 382, 135–147 (2011)
M. Witczak, R. Ziobro, L. Juszczak, J. Korus, Starch and starch derivatives in gluten-free systems—a review. J. Cereal Sci. 67, 46–57 (2016)
A. Olad, F.F. Azhar, M. Shargh, S. Jharfi, Application of response surface methodology for modeling of reactive dye removal from solution using starch-montmorillonite/polyaniline nanocomposite. Polym. Eng. Sci. 54, 1595–1607 (2014)
P. Ren, T. Shen, F. Wang, X. Wang, Z. Zhang, Study on biodegradable starch/OMMT nanocomposites for packaging applications. J. Polym. Environ. 17, 203 (2009)
H. Almasi, B. Ghanbarzadeh, A.A. Entezami, Physicochemical properties of starch–CMC–nanoclay biodegradable films. Int. J. Biol. Macromol. 46, 1–5 (2010)
F. Chivrac, E. Pollet, P. Dole, L. Avérous, Starch-based nano-biocomposites: plasticizer impact on the montmorillonite exfoliation process. Carbohydr. Polym. 79, 941–947 (2010)
R. Karthik, S. Meenakshi, Synthesis, characterization and Cr(VI) uptake study of polyaniline coated chitin. Int. J. Biol. Macromol. 72, 235–242 (2015)
R. Karthik, S. Meenakshi, Removal of Cr(VI) ions by adsorption onto sodium alginate-polyaniline nanofibers. Int. J. Biol. Macromol. 72, 711–717 (2015)
B. Qiu, C. Xu, D. Sun, H. Yi, J. Guo, X. Zhang, H. Qu, M. Guerrero, X. Wang, N. Noel, Polyaniline coated ethyl cellulose with improved hexavalent chromium removal. ACS Sustain. Chem. Eng. 2, 2070–2080 (2014)
L. Chuecas, J. Riley, The spectrophotometric determination of chromium in sea water. Anal. Chim. Acta 35, 240–246 (1966)
C. Dispenza, C.L. Presti, C. Belfiore, G. Spadaro, S. Piazza, Electrically conductive hydrogel composites made of polyaniline nanoparticles and poly (N-vinyl-2-pyrrolidone). Polymer 47, 961–971 (2006)
A. Olad, B. Naseri, Preparation, characterization and anticorrosive properties of a novel polyaniline/clinoptilolite nanocomposite. Prog. Org. Coat. 67, 233–238 (2010)
H. Liu, D. Chaudhary, S.-I. Yusa, M.O. Tadé, Glycerol/starch/Na + -montmorillonite nanocomposites: a XRD, FTIR, DSC and 1 H NMR study. Carbohydr. Polym. 83, 1591–1597 (2011)
H. Namazi, A. Dadkhah, M. Mosadegh, New biopolymer nanocomposite of starch-graft polystyrene/montmorillonite clay prepared through emulsion polymerization method. J. Polym. Environ. 20, 794–800 (2012)
V. Janaki, K. Vijayaraghavan, B.-T. Oh, K.-J. Lee, K. Muthuchelian, A. Ramasamy, S. Kamala-Kannan, Starch/polyaniline nanocomposite for enhanced removal of reactive dyes from synthetic effluent. Carbohydr. Polym. 90, 1437–1444 (2012)
D. Chaudhuri, D. Sarma, BF 3-doped polyaniline: a novel conducting polymer. Pramana 67, 135–139 (2006)
H.X. Huang, K.K. Yang, Y.Z. Wang, X.L. Wang, J. Li, Synthesis, characterization, and thermal properties of a novel pentaerythritol-initiated star-shaped poly (p-dioxanone). J. Polym. Sci., Part A: Polym. Chem. 44, 1245–1251 (2006)
A.G. Yavuz, E. Dincturk-Atalay, A. Uygun, F. Gode, E. Aslan, A comparison study of adsorption of Cr(VI) from aqueous solutions onto alkyl-substituted polyaniline/chitosan composites. Desalination 279, 325–331 (2011)
P. Müller, É. Kapin, E. Fekete, Effects of preparation methods on the structure and mechanical properties of wet conditioned starch/montmorillonite nanocomposite films. Carbohydr. Polym. 113, 569–576 (2014)
T.-T. Tee, L.T. Sin, R. Gobinath, S.-T. Bee, D. Hui, A. Rahmat, Q. Fang, Investigation of nano-size montmorillonite on enhancing polyvinyl alcohol–starch blends prepared via solution cast approach. Compos. B Eng. 47, 238–247 (2013)
S. Baral, N. Das, G.R. Chaudhury, S. Das, A preliminary study on the adsorptive removal of Cr(VI) using seaweed, Hydrilla verticillata. J. Hazard. Mater. 171, 358–369 (2009)
K.Z. Elwakeel, Removal of Cr(VI) from alkaline aqueous solutions using chemically modified magnetic chitosan resins. Desalination 250, 105–112 (2010)
V. Uskoković, Composites comprising cholesterol and carboxymethyl cellulose. Colloids Surf. B 61, 250–261 (2008)
A. Rashidzadeh, A. Olad, Novel polyaniline/poly (vinyl alcohol)/clinoptilolite nanocomposite: dye removal, kinetic, and isotherm studies. Desalination Water Treat. 51, 7057–7066 (2013)
M.R. Samani, S.M. Borghei, A. Olad, M.J. Chaichi, Removal of chromium from aqueous solution using polyaniline–poly ethylene glycol composite. J. Hazard. Mater. 184, 248–254 (2010)
M. Akram, H.N. Bhatti, M. Iqbal, S. Noreen, S. Sadaf, Biocomposite efficiency for Cr (VI) adsorption: kinetic, equilibrium and thermodynamics studies. J. Environ. Chem. Eng. 5, 400–411 (2017)
T.A. Saleh, A.M. Muhammad, S.A. Ali, Synthesis of hydrophobic cross-linked polyzwitterionic acid for simultaneous sorption of Eriochrome black T and chromium ions from binary hazardous waters. J. Colloid Interface Sci. 468, 324–333 (2016)
R. Karthik, S. Meenakshi, Facile synthesis of cross linked-chitosan–grafted-polyaniline composite and its Cr(VI) uptake studies. Int. J. Biol. Macromol. 67, 210–219 (2014)
V. Janaki, M.-N. Shin, S.-H. Kim, K.-J. Lee, M. Cho, A. Ramasamy, B.-T. Oh, S. Kamala-Kannan, Application of polyaniline/bacterial extracellular polysaccharide nanocomposite for removal and detoxification of Cr(VI). Cellulose 21, 463–472 (2014)
I. Langmuir, The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40, 1361–1403 (1918)
P.A. Kumar, S. Chakraborty, M. Ray, Removal and recovery of chromium from wastewater using short chain polyaniline synthesized on jute fiber. Chem. Eng. J. 141, 130–140 (2008)
R. Foroutan, R. Mohammadi, B. Ramavandi, M. Bastanian, Removal characteristics of chromium by activated carbon/CoFe2O4 magnetic composite and Phoenix dactylifera stone carbon. Korean J. Chem. Eng. 35, 2207–2219 (2018)
Y. Zheng, W. Wang, D. Huang, A. Wang, Kapok fiber oriented-polyaniline nanofibers for efficient Cr(VI) removal. Chem. Eng. J. 191, 154–161 (2012)
R. Nithya, T. Gomathi, P. Sudha, J. Venkatesan, S. Anil, S.-K. Kim, Removal of Cr(VI) from aqueous solution using chitosan-g-poly (butyl acrylate)/silica gel nanocomposite. Int. J. Biol. Macromol. 87, 545–554 (2016)
E.M. Kalhori, K. Yetilmezsoy, N. Uygur, M. Zarrabi, R.M.A. Shmeis, Modeling of adsorption of toxic chromium on natural and surface modified lightweight expanded clay aggregate (LECA). Appl. Surf. Sci. 287, 428–442 (2013)
A. Olad, F. Farshi Azhar, A study on the adsorption of chromium (VI) from aqueous solutions on the alginate-montmorillonite/polyaniline nanocomposite. Desalination and Water Treat. 52, 2548–2559 (2014)
Acknowledgements
The financial support of this research by the University of Tabriz is gratefully acknowledge.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Olad, A., Bastanian, M. & Bakht Khosh Hagh, H. Thermodynamic and Kinetic Studies of Removal Process of Hexavalent Chromium Ions from Water by Using Bio-conducting Starch–Montmorillonite/Polyaniline Nanocomposite. J Inorg Organomet Polym 29, 1916–1926 (2019). https://doi.org/10.1007/s10904-019-01152-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01152-w