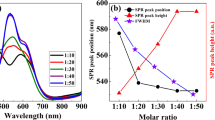
Abstract
The emergence and spread of pathogenic microbes with resistance to multiple antibiotics necessitates the development of new broad-spectrum microbicides. Metal nanoparticles are one such microbicide and they have been recognized for their potential value in fighting harmful microbes. In this work, we show the preparation and antimicrobial characterization of copper nanoparticles, with a small percentage of copper (I) oxide, synthesized by a chemical method based on a bottom-up approach in a nonaqueous medium. In particular, we developed a new route to stabilize the copper nanoparticles, synthesized in ethanol, using an aminosilane as a capping agent. The particles were later centrifuged and suspended in ethylene glycol. The morphology, structure and stability of the Cu-APTMS NPs were characterized by UV–Vis and FTIR spectroscopy, TEM, AFM and GI-XRD techniques. The presence of colloidal nanoparticles was found 4 months after synthesization and a characteristic absorption LSPR band was registered in the UV–Vis spectrum. The Cu-APTMS NPs showed a significant in vitro degradation activity against bacterial DNA, which is important in vivo microbicidal activity. The Cu-APTMS NPs showed a strong bactericidal effect against planktonic forms of Gram-negative (Pseudomonas aeruginosa and enterohemorrhagic Escherichia coli) and Gram-positive (Staphylococcus aureus and Listeria monocytogenes) bacteria. This bactericidal effect was also observed to severely limit the viability and germination proficiency of spores of the food-poisoning and gas-gangrene producer Clostridium perfringens. In addition, pathogenic fungi (Candida tropicalis and Fusarium verticillioides) were irreversibly deactivated by treatment with Cu-APTMS NPs.







Similar content being viewed by others
References
S. Guo, E. Wang, Noble metal Nanomaterials: controllable synthesis and application in fuel cells and analytical sensors. Nano Today 6, 240–264 (2011)
Y. Yan, S.C. Warren, P. Fuller, B.A. Grzybowski, Chemoelectronic circuits based on metal nanoparticles. Nat. Nanotechnol. 11, 603–608 (2016)
R.A. Potyrailo, Toward high value sensing: monolayer-protected metal nanoparticles in multivariable gas and vapor sensors. Chem. Soc. Rev. 46, 5311–5346 (2017)
C. Shen, C. Hui, T. Yang, C. Xiao, J. Tian, L. Bao, S. Chen, H. Ding, H. Gao, Monodisperse noble-metal nanoparticles and their surface enhanced raman scattering properties. Chem. Mater. 20, 6939–6944 (2008)
G. Prieto, J. Zecevic, H. Friedrich, K. Jong, P. Jongh, Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 12, 34–39 (2013)
B. Roldan Cuenya, Synthesis and catalytic properties of metal nanoparticles: size, shape, support, composition, and oxidation state effects. Thin Solid Films 518, 3127–3150 (2010)
S.M. Dizaj, F. Lotfipoura, M. Barzegar-Jalali, M.H. Zarrintana, K. Adibkiab, Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 44, 278–284 (2014)
F. Parveen, B. Sannakki, M. Mandke, H. Pathan, Copper nanoparticles: synthesis methods and its light harvesting performance. Solar Energy Mater. Solar Cells 144, 371–382 (2016)
D. Deng, Y. Jin, Y. Cheng, T. Qi, F. Xiao, Copper nanoparticles: aqueous phase synthesis and conductive films fabrication at low sintering temperature. ACS Appl. Mater. Interfaces 5, 3839–3846 (2013)
S. Jeong, S.H. Lee, Y. Jo, S.S. Lee, Y. Seo, B. Ahn, G. Kim, G. Jang, J. Park, B. Ryu, Y. Choi, Air-stable, surface-oxide free Cu nanoparticles for highly conductive Cu ink and their application to printed graphene transistors. J. Mater. Chem. C. 1, 2704–2710 (2013)
Y. Guo, F. Cao, X. Lei, L. Mang, S. Cheng, J. Song, Fluorescent copper nanoparticles: recent advances in synthesis and applications for sensing metal ions. Nanoscale 8, 4852–4863 (2016)
T. Ramani, K. Prasant, B. Sreedhar, Air stable colloidal copper nanoparticles: synthesis, characterization and their surface-enhanced Raman scattering properties. Phys. E 77, 65–71 (2016)
M. Gawande, A. Goswami, F. Felpin, T. Asefa, X. Huang, R. Silva, X. Zou, R. Zborl, R. Varma. Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem. Rev. 116, 3722–3811 (2016)
T. Kruk, K. Szczepanowicz, J. Stefanska, R. Socha. P. Warszynski, Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Colloids Surf. B 128, 17–22 (2015)
L. Duran Pachon, G. Rothenberg, Transition-metal nanoparticles: synthesis, stability and the leaching issue. Appl. Organometal. Chem. 22, 288–299 (2008)
T. Dang-Bao, C. Pradel, I. Favier, M. Gómez, Making copper(0) nanoparticles in glycerol: a straightforward synthesis for a multipurpose catalyst. Adv. Synth. Catal. 359, 2832–2846 (2017)
B.H. Patel, M.Z. Channiwala, S.B. Chaudhari, A.A. Mandot, Biosynthesis of copper nanoparticles; its characterization and efficacy against human pathogenic bacterium. J. Environ. Chem. Eng. 4, 2163–2169 (2016)
L. Esteban-Tejeda, F. Malpartida, A. Esteban-Cubillo, C. Pecharromán, J.S. Moya. Antibacterial and antifungal activity of a soda-lime glass containing copper nanoparticles. Nanotechnology. 20, 505701 (2009)
Y.W. Baek, Y.J. An, Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO and Sb2O3) to Escherichia coli, Bacillus subtilis and Streptococcus aureus. Sci. Total Envirom. 409, 1603–1608 (2011)
A. Azam, A.S. Ahmed, M. Oves, M.S. Khan, A. Memic, Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int. J. Nanomed. 7, 3527–3535 (2012)
A.K. Chatterjee, R.K. Sarkar, A.P. Chattopadhyay, P. Aich, R. Chakraborty, T. Basu, A simple robust method for synthesis of metallic copper nanoparticles of high antibacterial potency against E. coli. Nanotechnology. 23, 085103 (2012)
M.S. Hassan, T. Amna, O.B. Yang, M.H. El-Newehy, S.S. Al-Deyab, M.S. Khil. Smart copper oxide nanocrystals: synthesis, characterization, electrochemical and potent antibacterial activity. Colloids Surf. B. Biointerfaces. 97, 201–206 (2012)
A. Pramanik, D. Laha, D. Bhattacharya, P. Pramanik, P. Karmakar, A novel study on antibacterial activity of copper iodide nanoparticles mediated by DNA and membrane damage. Colloids Surf. B. Biointerfaces. 96, 50–55 (2012)
K. Giannousi, K. Lafazanis, J. Arvanitidis, A. Pantazaki, C. Dendrinou-Samara, Hydrothermal synthesis of copper based nanoparticles: antimicrobial screening and interaction with DNA. J. Inorg. Biochem. 133, 24–32 (2014)
D. Yohan, D. Chithrani, Applications of nanoparticles in nanomedicine. J. Biomed. Nanotechnol. 10, 2371–2392 (2014)
T.V. Duncan, Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 363, 1–24 (2011)
J.A. Lemire, J.J. Harrison, S.P. Turner, Antimicrobial activity of metals, mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 11, 371–384 (2016)
A. Stacy, L. McNally, S.E. Darch, S.P. Brown, M. Whiteley, The biogeography of polymicrobial infection. Nat. Rev. Microbiol. 14, 93–105 (2016)
A.N. Kremer, H.J. Hoffmann, Substractive hybridization yields a silver resistance determinant unique to nosocomial pathogens in the Enterobacter cloacae complex. J. Clin. Microbiol. 50, 3249–3257 (2012)
L.L. Maragakis, E.N. Perencecich, S.E. Cosgrove, Clinical and economic burden of antimicrobial resistance. Expert Rev. Anti-Infect. Ther. 5, 751–763 (2008)
A. Alanis, Resistance to antibiotics: are we in the post-antibiotic era? Arch. Med. Res. 36, 697–705 (2005)
R. Laxminarayan, A. Duse, C. Wattal, A.K.M. Zaidi, F. Heiman, L. Wertheim, N. Sumpradit, E. Vlieghe et al., Antimicrobial resistance the need for global solutions. Lancet Inf. Dis. 12, 1057–1098 (2013)
T.D. Gootz, The global problem of antibiotic resistance. Crit. Rev. Immunol. 30, 79–93 (2010)
CDC, Antibiotic resistance threats in the United States (Center for Disease Control and Prevention, Atlanta, 2013)
U. Theuretzbacher, Antibiotic innovation for future public health needs. Clin. Microbiol. Infect. 23, 713–717 (2017)
S. Rossiter, M. Fletcher, W. Wuest, Natural products as platforms to overcome antibiotic resistance. Chem. Rev. 117, 12415–12474 (2017)
V. Challinor, H. Bode, Bioactive natural products from novel microbial sources. Ann. NY. Acad. Sci. 1354, 82–97 (2015)
T. Rahman, B. Yarnall, D. Doyle, Efflux drug transporters at the forefront of antimicrobial resistance. Eur. Biophys. J. 46, 647–653 (2017)
S. Correia, P. Poeta, M. Hébraud, J.L. Capelo, G. Igrejas, Mechanisms of quinolone action and resistance: where do we stand? J. Med. Microbiol. 66, 551–559 (2017)
D. Dar, R. Sorek, Regulation of antibiotic-resistance by non-coding RNA in bacteria. Curr. Opin. Microbiol. 36, 111–117 (2017)
N. Hѳiby, T. Bjarnsholt, M. Givskov, S. Molinc, O. Ciofub, Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agent. 35, 322–332 (2010)
A.K. Thabit, J.L. Crandon, D.P. Nicolau, Antimicrobial resistance: impact on clinical and economical outcomes and the need for new antimicrobials. Expert Opin. Pharmacother. 2, 159–177 (2015)
J.N. Slavin, J. Asnis, U.O. Häfeli, H. Bach, Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 15, 65 (2017)
F.N. Oktar, M. Yetmez, D. Ficai, A. Ficai, F. Dumitru, A. Pica, Molecular mechanisms and targets of the antimicrobial activity of metal nanoparticles. Curr. Top. Med. Chem. 15, 1583–1588 (2015)
L. Wang, C. Hu, L. Shao, The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomed. 12, 1227–1249 (2017)
G.R. Rudamurthy, M.K. Swamy, U.R. Sinniah, A. Ghasemzadeh, Nanoparticles: alternatives against drug-resistant pathogenic microbes. Molecules 21, 836 (2016)
M. Vincent, R.E. Duval, P. Hartemann, M. Engels-Deutsch, Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 124, 1032–1046 (2017)
H. Palza, M. Nuñez, R. Bastías, K. Delgado, In situ antimicrobial behavior of materials with copper-based additives in a hospital environment. Int. J. Antimicrob. Agents 51, 912–917 (2018)
T.M. Dung Dang, T.T. Tuyet Le, E. Fribourg-Blanc, M. Chien Dang, The influence of solvents and surfactants on the preparation of copper nanoparticles by a chemical reduction method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2, 025004 (2011)
P. Singh, Y. Kim, D. Zhang, D. Yang, Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 34, 588–599 (2016)
N. Pantidos, M.C. Edmundson, L. Horsfall, Room temperature bioproduction, isolation and anti-microbial properties of stable elemental copper nanoparticles. New Biotechnol. 40, 275–281 (2018)
N. Nagar, V. Devra, Green synthesis and characterization of copper nanoparticles using Azadirachta indica leaves. Mater. Chem. Phys. 213, 44–51 (2018)
N. Sreeju, A. Rufus, D. Philip, Microwave–assisted rapid synthesis of copper nanoparticles with exceptional stability and their multifaceted applications. J. Mol. Liq. 221, 1008–1021 (2016)
G.H. Hong, S.W. Kang, Synthesis of monodisperse copper nanoparticles by utilizing 1-butyl-3-methylimidazolium nitrate and its role as counteranion in ionic liquid in the formation of nanoparticles. Ind. Eng. Chem. Res. 52, 794–797 (2013)
C. Schmadicke, M. Poetschke, L.D. Renner, L. Baraban, M. Bobeth, G. Cuniberti, Copper nanowire synthesis by directed electrochemical nanowire assembly. RSC Adv 4, 46363–46368 (2014)
M.H. Kang, S.J. Lee, J.Y. Park, J.K. Park, Carbon-coated copper nanoparticles: Characterization and fabrication via ultrasonic irradiation. J. Alloys Compd. 735, 2162–2166 (2018)
A.R. Sadrolhosseini, A.S.B.M. Noor, K. Shameli, G. Mamdoohi, M.M. Moksin, M.A. Mahdi, Laser ablation synthesis and optical properties of copper nanoparticles. J. Mater. Res. 28, 2629–2636 (2013)
A. Kumar, A. Saxena, A. De, R. Shankar, S. Mozumdar, Facile synthesis of size-tunable copper and copper oxide nanoparticles using reverse microemulsions. RSC Adv. 3, 5015–5021 (2013)
A. Wang, L. Chen, F. Xu, Z. Yan, In-situ synthesis of copper nanoparticles within ionic liquid–in—vegetable oil microemulsions and their direct use as high efficient nanolubricants. RSC Adv. 4, 45251–45257 (2014)
L. Xu, J.H. Peng, C. Srinivasakannan, L.B. Zhang, D. Zhang, C. Liu, S.X. Wang, A.Q. Shen. Synthesis of copper nanoparticles by a T-shaped microfluidic device. RSC Adv. 4, 25155–25159 (2014)
Q. Liu, D. Zhou, Y. Yamamoto, R. Ichino, M. Okido, Preparation of Cu nanoparticles with NaH4 by aqueous reduction method. Trans. Nonferrous Met. Soc. China 22, 117–123 (2012)
K. Liu, Y. Song, S. Chen, Electrocatalytic activities of alkyne-functionalized copper nanoparticles in oxygen reduction in alkaline. Media. J. Power Sources 268, 469–475 (2014)
Y. Hokita, M. Kanzaki, T. Sugiyama, R. Arakawa, H. Kawasaki, High-concentration synthesis of sub-10-nm copper nanoparticles for application to conductive nanoinks. ACS Appl. Mater. Interfaces 7, 19382–19389 (2015)
H.X. Zhang, U. Siegert, R. Liu, W.B. Cai. Facile fabrication of ultrafine copper nanoparticles in organic solvent. Nanoscale Res. Lett. 4, 705–708 (2009)
B.K. Park, S. Jeong, D. Kim, J. Moon, S. Lim, J.S. Kim, Synthesis and size control of monodisperse copper nanoparticles by polyol method. J. Coll. Interface Sci. 311, 417–424 (2007)
M.V. Roldán, P. de Oña, Y. Castro, A. Durán, P. Faccendini, C. Lagier, R. Grau, N.S. Pellegri, Photocatalytic and biocidal activity of novel coating systems of mesoporous and dense TiO2-anatase containing silver nanoparticles. Mater. Sci. Eng. C 43, 630–640 (2014)
M.V. Roldán, Y. Castro, N. Pellegri, A. Durán, Enhanced photocatalytic activity of mesoporous TiO2 Sol-Gel coatings doped with Ag nanoparticles. J. Sol-Gel Sci. Technol. 76, 180–194 (2015)
C. Rodriguez-Abreu, M. Sánchez-Domínguez, Nanocolloids: A Meeting Point for Scientists and Technologists, 1st edn. (Elsevier, 2016), pp. 159
L. Liz-Marzán, M. Giersig, P. Mulvaney, Synthesis of gold-silica core-shell particles. Langmuir. No. 18 12, 4329–4335 (1996)
P. Pongwan, K. Wetchakun, S. Phanichphan, N. Wetchakun, Enhancement of visible-light photocatalytic activity of Cu-doped TiO2 nanoparticles. Res. Chem. Intermed. 42, 4 (2015)
X.-M. Zhu, Y.-X.J. Wang, K.C.-F. Leung, S.-F. Lee, F. Zhao, D.-W. Wang, J.M. Lai, C. Wan, C.H. Cheng, A.T. Ahuja, Enhanced cellular uptake of aminosilane-coated superparamagnetic iron oxide nanoparticles in mammalian cell lines. Int. J. Nanomed. 7, 953–964 (2012)
A.L. Arabolaza, A. Nakamura, M.E. Pedrido, L. Martelotto, L. Orsaria, R.R. Grau. Characterization of a novel inhibitory feeback of the anti-anti-sigma SpoIIA on Spo0A activation during development in Bacillus subtilis. Mol. Microbiol. 47, 1251–1263 (2003)
M.B. Méndez, A. Goñi, W. Ramirez, R.R. Grau. Sugar inhibits the production of the toxins that trigger clostridial gas gangrene. Microb. Pathog. 52, 85–91 (2012)
R. Grau, D. Gardiol, G.C. Glikin, D. de Mendoza, DNA supercoiling and thermal regulation of unsaturated fatty acid synthesis in Bacillus subtilis. Mol. Microbiol. 11, 933–941 (1994)
V.A. Philippe, M.B. Méndez, I.H. Huang, L.M. Orsaria, M.R. Sarker, R.R. Grau. Inorganic phosphate induces spore morphogenesis and enterotoxin production in the intestinal pathogen Clostridium perfringens. Infect. Immun. 74, 3651–3656 (2006)
T. Igarashi, P. Setlow, Interaction between individual protein components of the GerA and GerB nutrient receptors that trigger germination on Bacillus subtilis spores. J. Bacteriol. 187, 2514–2518 (2005)
D.V. Ravi Kumar, I. Kim, Z. Zhong, K. Kim, D. Lee, J. Moon, Cu(II)-alkyl amine complex mediated hydrothermal synthesis of Cu nanowires: exploring the dual role of alkyl amines. Phys. Chem. Chem. Phys. 16, 22107 (2014)
K. Rice, A. Paterson, M. Stoykovich, Nanoscale Kirkendall effect and oxidation kinetics in copper nanocrystals characterized by real-time, in situ optical spectroscopy, Part. Part. Syst. Charact. 32, 1–8 (2014)
L. Lutterotti, Total pattern fitting for the combined size-strain-stress-texture determination in thin film diffraction. Nuclear Inst. Methods Phys. Res. B 268, 334–340 (2010)
C. Salzemann, A. Brioude, M.-P. Pileni, Tuning of copper nanocrystals optical properties with their shapes. J. Phys. Chem. B 110, 7208–7212 (2006)
S. Bhattacharjee, DLS and zeta potential-What they are and what they are not? J. Control. Release 235, 337–351 (2016)
G. Socrates, Infrared and Raman Characteristic Group Frequencies, 3rd. edn. (John Wiley & Sons Ltd., Chichester, 2001), p. 145
L. Téllez, F. Rubio, R. Peña-Alonso, J. Rubio, Seguimiento por espectroscopia infrarroja (FT-IR) de la copolimerización de TEOS (tetraetilortosilicato) y PDMS (polidimetilsiloxano) en presencia de tbt (tetrabutiltitanio). Bol. Soc. Esp. Ceram. 43, 883–890 (2004)
M.V. Roldán, N.S. Pellegri, O.A. de Sanctis, Optical response of silver nanoparticles stabilized by amines to LSPR based sensors. Proc. Mater. Sci. 1, 594–600 (2012)
R.C. Rodríguez, L. Yate, E. Coy, ÁM. Martínez-Villacorta, A.V. Bordonia, S. Moya, P.C. Angelomé, Copper nanoparticles synthesis in hybrid mesoporous thin films: controlling oxidation state and catalytic performance through pore chemistry. Appl. Surf. Sci. 471, 862–868 (2019)
V. Donato, F. Rodríguez Ayala, S. Cogliati, C. Bauman, J.G. Costa, C. Leñini, R. Grau, Bacillus subtilis biofilm extends Caenorhabditis elegans longevity through downregulation of the insulin-like signaling pathway. Nat Commun. 8, 14332 (2017)
M. Mendez, I. Huang, K. Ohtani, R. Grau, T. Shimizu, M.R. Sarker, Carbon catabolite repression of type IV pilus-dependent gliding motility in the anaerobic pathogen Clostridium perfringens. J. Bacteriol. 190, 48–60 (2008)
J.M. Rangel, P.H. Sparling, C. Crowe, P.M. Griffin, D.L. Swerdlow, Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg. Infect. Dis. 11, 603–609 (2005)
J.A. Vázquez-Boland, M. Kuhn, P. Berche, T. Chakraborty, G. Domínguez-Bernal, W. Goebel, B. González-Zorn, J. Wehland, J. Kreft, Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14, 584–640 (2001)
Y.D. Bakthavatchalam, L.E. Nabarro, B. Veeraraghavan, Evolving rapid methicillin-resistant Staphylococcus aureus detection: cover all the bases. J. Glob. Infect. Dis. 9, 18–22 (2017)
A.K. Chatterjee, R. Chakraborty, T. Basu, Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 25, 135101 (2014)
T. Maniatis, E.F. Fritsch, J. Sambrook. Molecular cloning: a laboratory manual. (Cold Spring Harbor Laboratory, New York, 545, 1982). ISBN 0-87969-136-0
A. Bonici, G. Lusvardi, G. Malavasi, L. Menabue, A. Piva, Synthesis and characterization of bioactive glasses functionalized with Cu nanoparticles and organic molecules. J. Eur. Ceram. Soc. 32, 2777–2783 (2012)
V. Ainaa, G. Cerrato, G. Martra, G. Malavasid, G. Lusvardid, L. Menabue, Towards the controlled release of metal nanoparticles from biomaterials: physico-chemical, morphological and bioactivity features of Cu-containing sol-gel glasses. Appl. Surf. Sci. 283, 240–248 (2013)
S. Jadhav, S. Gaikwad, M. Nimse, A. Rajbhoj, Copper oxide nanoparticles: synthesis, characterization and their antibacterial activity. J. Clust. Sci. 22, 121–129 (2011)
M. Hans, A. Erbe, S. Mathews, Y. Chen, M. Solioz, F. Mücklich. Role of copper oxides in contact killing of bacteria. Langmuir 29, 16160–16166 (2013)
O. Akhavan, E. Ghaderi, Cu and CuO nanoparticles immobilized by silica thin films as antibacterial materials and photocatalysts. Surf. Coat. Technol. 205, 219–223 (2010)
S. Cogliati, J.G. Costa, F. Rodriguez Ayala, V. Donato, R. Grau, Bacterial spores and its relatives as agents of mass destruction. J. Bioterror. Biodef 7, 141 (2016)
M.J. Leggett, G. McDonnell, S.P. Denyer, P. Setlow, J.Y. Maillard, Bacterial spore structures and their protective role in biocide resistance. J. Appl. Microbiol. 113, 485–498 (2012)
Acknowledgements
The authors thank the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP-2013-0553) and the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, PICT 20103-423 and PICT 2012-2577) for the financial support. We also thank Dra. Renata Strubbia for the assistance in acquiring the TEM images and Dr. Nestor Delorenzi for the use of the zeta potential analizer.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Porta, E., Cogliati, S., Francisco, M. et al. Stable Colloidal Copper Nanoparticles Functionalized with Siloxane Groups and Their Microbicidal Activity. J Inorg Organomet Polym 29, 964–978 (2019). https://doi.org/10.1007/s10904-018-01071-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-018-01071-2