Abstract
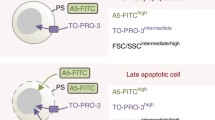
Apoptosis is the programmed cell death pathway that is critical for maintaining homeostasis, in which cancer cells can evade to ensure survival. For pharmaceutical drug discovery, it is important to characterize and compare different cancer therapeutics (i.e., small molecules, antibody drugs, cell therapies) that can initiate the process of apoptosis, enabling the identification of potential therapeutic candidates. In this work, we developed and demonstrated a multiplex detection method for monitoring apoptosis and necrosis with Annexin V, Caspase-3, and Propidium Iodide (PI) using the Cellaca® PLX Image Cytometer (Revvity Health Sciences, Inc., Lawrence, MA). First, apoptosis was induced in Jurkat and K562 cell lines with staurosporine over the course of 24 h, where apoptosis and necrosis were assessed at 0, 1, 1.5, 2, 4, 20, and 24 h timepoints. Samples were stained with Hoechst 33342 (total dye), Annexin V-APC (early-stage apoptosis), Caspase-3 488 (late-stage apoptosis), and PI (necrosis) at each timepoint and evaluated using image cytometry. Results showed that apoptotic factors and cascades were successfully detected along the pathway from early- to late-stage apoptosis, and ultimately necrosis. A clear trend was observed analyzing apoptotic and necrotic populations during the first 1.5 h, showing differences of up to ~15% in single Annexin V+ and Caspase-3+ populations in treated Jurkat cells, however, a significant increase in double positive apoptotic/necrotic cells for Annexin V+PI+ and Capase-3+PI+ was not observed until 20 h. Upon further analysis between apoptotic populations only, Annexin V+ only populations were higher than Caspase-3+ only populations by up to ~20% between 0 and 1.5 h. Conversely, K562 cells did not exhibit a notable change in apoptotic and necrotic populations due to low sensitivity to staurosporine. The proposed image cytometric detection method may provide an effective and efficient tool for rapid and reliable simultaneous detection of early- late-stage apoptosis, and necrosis. Therefore, allowing researchers to better characterize and screen potential cancer therapeutic drug candidates for their treatment efficacy in a higher throughput manner.






Similar content being viewed by others
Data Availability
Data is provided within the manuscript or supplementary information files.
References
Cavalcante GC, Schaan AP, Cabral GF et al (2019) A cell’s fate: an overview of the Molecular Biology and Genetics of Apoptosis. Int J Mol Sci 20:4133
McIlwain DR, Berger T, Mak TW (2024) Caspase functions in cell death and disease, vol 16. Cold Spring Harbor Perspectives in Biology
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Kumar R, Herbert PE, Warrens AN (2005) An introduction to death receptors in apoptosis. Int J Surg 3:268–277
Galluzzi L, Vitale I, Aaronson SA et al (2018) Molecular mechanisms of cell death: recommendations of the nomenclature Committee on Cell Death 2018. Cell Death & Differentiation 25:486–541
Tummers B, Green DR (2017) Caspase-8: regulating life and death. Immunol Rev 277:76–89
Kantari C, Walczak H (2011) Caspase-8 and bid: Caught in the act between death receptors and mitochondria. Biochimica et Biophysica Acta (BBA) -. Mol Cell Res 1813:558–563
Jan R, Chaudhry G-e-S (2019) Understanding apoptosis and apoptotic pathways targeted Cancer therapeutics. Adv Pharm Bull 9:205–218
Chipuk JE, Bouchier-Hayes L, Green DR (2006) Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death & Differentiation 13:1396–1402
Logue SE, Elgendy M, Martin SJ (2009) Expression, purification and use of recombinant annexin V for the detection of apoptotic cells. Nat Protoc 4:1383–1395
Chaurio RA, Janko C, Muñoz LE, Frey B, Herrmann M, Gaipl US (2009) Phospholipids: key players in apoptosis and Immune Regulation. Molecules 14:4892–4914
Demchenko AP (2013) Beyond annexin V: fluorescence response of cellular membranes to apoptosis. Cytotechnology 65:157–172
Eskandari E, Eaves CJ (2022) Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J Cell Biol 221:e202201159
Rathore R, McCallum JE, Varghese E, Florea A-M, Büsselberg D (2017) Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis 22:898–919
Mu B, Yang J-l, Gou L-t et al (2011) Polyclonal rabbit anti-murine plasmacytoma cell globulins induce myeloma cells apoptosis and inhibit tumour growth in mice. Apoptosis 16:370–381
Pfeffer CM, Singh ATK (2018) Apoptosis: a target for Anticancer Therapy. Int J Mol Sci 19:448
Gordon JL, Brown MA, Reynolds MM (2018) Cell-based methods for determination of efficacy for candidate therapeutics in the Clinical Management of Cancer. Diseases 6:85
Woynarowska BA, Woynarowski JM (2002) Preferential targeting of apoptosis in tumor versus normal cells. Biochimica et Biophysica Acta (BBA) -. Mol Basis Disease 1587:309–317
Chan LL, Lyettefi EJ, Pirani A, Smith T, Qiu J, Lin B (2011) Direct concentration and viability measurement of yeast in corn mash using a novel imaging cytometry method. J Ind Microbiol Biotechnol 38:1109–1115
Kari S, Subramanian K, Altomonte IA, Murugesan A, Yli-Harja O, Kandhavelu M (2022) Programmed cell death detection methods: a systematic review and a categorical comparison. Apoptosis 27:482–508
Drescher H, Weiskirchen S, Weiskirchen R (2021) Flow Cytometry: a blessing and a curse. Biomedicines 9:1613
Lekshmi A, Varadarajan SN, Lupitha SS et al (2017) A quantitative real-time approach for discriminating apoptosis and necrosis. Cell Death Discovery 3
Zhu X, Fu A, Luo KQ (2012) A high-throughput fluorescence resonance energy transfer (FRET)-based endothelial cell apoptosis assay and its application for screening vascular disrupting agents. Biochem Biophys Res Commun 418:641–646
Leavesley SJ, Rich TC (2016) Overcoming limitations of FRET measurements. Cytometry Part A 89:325–327
Chan LL-Y, Lai N, Wang E, Smith T, Yang X, Lin B (2011) A rapid detection method for apoptosis and necrosis measurement using the Cellometer imaging cytometry. Apoptosis 16:1295–1303
Nitta CF, Pierce M, Hem S et al (2023) Cellaca® PLX image cytometer as an alternative for immunophenotyping, GFP/RFP transfection efficiencies, and apoptosis analysis. Analytical Biochemistry
Lema C, Varela-Ramirez A, Aguilera RJ (2011) Differential nuclear staining assay for high-throughput screening to identify cytotoxic compounds. Curr Cell Biochem 1:1–14
Ouyang L, Shi Z, Zhao S et al (2012) Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif 45:487–498
Malsy M, Bitzinger D, Graf B, Bundscherer A (2019) Staurosporine induces apoptosis in pancreatic carcinoma cells PaTu 8988t and Panc-1 via the intrinsic signaling pathway. Eur J Med Res 24
Aguilar-Lemarroy A, Romero-Ramos JE, Olimon-Andalon V et al (2008) Apoptosis induction in Jurkat cells and sCD95 levels in women’s sera are related with the risk of developing cervical cancer. BMC Cancer 8
Fulda S (2009) Tumor resistance to apoptosis. Int J Cancer 124:511–515
Sundquist T, Moravec R, Niles A, O’Brien M, Riss T (2006) TIMING YOUR APOPTOSIS ASSAYS. Cell Notes
Riss TL, O’Brien MA, Moravec RA, Kupcho K, Niles AL (2021) Apoptosis marker assays for HTS. Eli Lilly & Company and the National Center for Advancing Translational Sciences
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Mackenzie Pierce, Yongyang Huang, Allen Lin, Carolina Franco Nitta, Dmitry Kuksin, Bo Lin, and Leo Li-Ying Chan contributed to the study conception and design. Material preparation, data collection and analysis were performed by Mackenzie Pierce, Yongyang Huang, Allen Lin, Carolina Franco Nitta, Dmitry Kuksin, Bo Lin, and Leo Li-Ying Chan. The first draft of the manuscript was written by Mackenzie Pierce and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript. All trademarks, service marks, trade names, and brand names are the property of their respective owners.
Corresponding author
Ethics declarations
Conflict of interest
The authors MP, YH, AL, CFN, DK, BL, and LLC declare competing financial interests. The work performed in the manuscript is for reporting on applications of an instrument from Revvity Health Sciences, Inc. (an indirect parent company of Nexcelom Biosciences, LLC.). The performed experiment is to demonstrate a multiplex fluorescence imaging methods for identifying early- and late-stage apoptosis.
Ethical Approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
10895_2024_3590_MOESM1_ESM.xlsx
Supplementary Material 1: Table 1 Staurosporine-treated Jurkat cell population percentage identification of Annexin V with respect to PI, Caspase-3 with respect to PI, and Annexin V with respect to Caspase-3. Cells showed an increase in apoptotic and necrotic populations as time in treatment advanced.
10895_2024_3590_MOESM2_ESM.xlsx
Supplementary Material 2: Table 2 Untreated control Jurkat cell population percentage identification of Annexin V with respect to PI, Caspase-3 with respect to PI, and Annexin V with respect to Caspase-3. Cells showed low levels of apoptotic and necrotic populations as time advanced.
10895_2024_3590_MOESM3_ESM.xlsx
Supplementary Material 3: Table 3 Staurosporine-treated K562 cell population percentage identification of Annexin V with respect to PI, Caspase-3 with respect to PI, and Annexin V with respect to Caspase-3. Cells showed low levels of apoptotic and necrotic populations as time in treatment advanced.
10895_2024_3590_MOESM4_ESM.xlsx
Supplementary Material 4: Table 4 Untreated control K562 cell population percentage identification of Annexin V with respect to PI, Caspase-3 with respect to PI, and Annexin V with respect to Caspase-3. Cells showed low levels of apoptotic and necrotic populations as time advanced.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pierce, M., Huang, Y., Lin, A. et al. A Multiplex Assay to Simultaneously Monitor Apoptosis and Necrosis Using the Cellaca® PLX Image Cytometer. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03590-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03590-3