Abstract
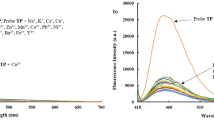
The condensation product (L) of 4,4′-methylenedianiline and p-anisaldehyde acts as colorimetric sensor for Cu2+ and Pb2+ ions. On interaction with Cu2+, ethanolic solution of L changes its color to brown while it becomes light pink on interaction with Pb2+. Interaction of Al3+ with L coated paper strip emits bright blue fluorescence. Metal ions like Mg2+, Cu2+, Li+, K+, Na+, Mn2+, Al3+, Hg2+, Co2+, Pb2+, Ni2+, Cd2+, Zn2+, Fe3+ do not interfere the paper strip sensor. The fluorescent intensity of L in ethanol is quenched 25 times by Pb2+ ion. The interaction between L and Pb2+ is reversible and the detection limit of Pb2+ is 10−6 M. The binding constant and stoichiometry of binding between L and Pb2+ was calculated to be 104.8 and 1:2. Theoretical calculations show that the binding of the metal ions to L are favorable and the fluorescence of L is due to π → π* transition.













Similar content being viewed by others
References
Gupta VK, Shoora SK, Kumawat LK, Jain AK (2015) A highly selective colorimetric and turn-on fluorescent chemosensor based on 1-(2-pyridylazo)-2-naphthol for the detection of aluminium(III) ions. Sensors Actuators B 209:15–24
Valeur B, Leray I (2000) Coord Chem Rev 205(1):3–40
Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE, Chem ACS (1997) Rev. 97(5):1515–1566
Carter KP, Young AM, Palmer AE, Chem ACS (2014) Rev. 114(8):4564–4601
L. Fabbrizzi, M. Licchelli, P. Pallavicini, A. Perotti, D. Sacchi, Angewandte Chemie. 33(19) (1994) 1975–1977
Chen D, Chen P, Zong L, Sun Y, Liu G, Yu X, Qin J (2017) R Soc Open Sci 4:171161
Needleman H (2004) Lead Poisoning. Annu Rev Med 55:209–222
Winder C, Carmichael NG, Lewis PD (1982) Effects of chronic low level lead exposure on brain development and function. Trends Neurosci 5:207–209
Sobin C, Parisi N, Schaub T, Gutierrez M, Ortega AX (2011) δ-Aminolevulinic Acid Dehydratase Single Nucleotide Polymorphism 2 and Peptide Transporter 2*2 Haplotype May Differentially Mediate Lead Exposure in Male Children. Arch Environ Contam Toxicol 61:521–529
Menezes JA, Viana GFD, Paes CR (2012) Determinants of lead exposure in children on the outskirts of Salvador, Brazil. Environ Monit Assess 184:2593–2603
Bowins RJ, McNutt RH (1994) Electrothermal isotope dilution inductively coupled plasma mass spectrometry method for the determination of sub-ng ml–1levels of lead in human plasma. J Anal At Spectrochem 9:1233–1236
Cave MR, Butler O, Cook JM, Cresser MS, Miles DL (2000) Environmental analysis. J Anal At Spectrom 15:181–235
Cave MR, Butler O, Chenery RN, Cook JM, Cresser MS, Miles DL (2001) Atomic Spectrometry Update. Environmental analysis. J Anal At Spectrom 16:194–235
Feldman BJ, Osterloh JD, Hata BH, D’Alessandro A (1994) Determination of Lead in Blood by Square Wave Anodic Stripping Voltammetry at a Carbon Disk Ultramicroelectrode. Anal Chem 66:1983–1989
Tahan JE, Granadillo VA, Romero RA (1994) Electrothermal atomic absorption spectrometric determination of Al, Cu, Fe, Pb, V and Zn in clinical samples and in certified environmental reference materials. Anal Chim Acta 295:187–197
Chen CT, Huang WP (2002) A Highly Selective Fluorescent Chemosensor for Lead Ions. J Am Chem Soc 124:6246–6247
Kavallieratos K, Rosenberg JM, Chen WZ, Ren T (2005) Fluorescent Sensing and Selective Pb(II) Extraction by a Dansylamide Ion-Exchanger. J Am Chem Soc 127:6514–6515
He Q, Miller EW, Wong AP, Chang CJ (2006) A Selective Fluorescent Sensor for Detecting Lead in Living Cells. J Am Chem Soc 128:9316–9317
Li CL, Liu KT, Lin YW, Chang HT (2011) Fluorescence Detection of Lead(II) Ions Through Their Induced Catalytic Activity of DNAzymes. Anal Chem 83:225–230
G Pina-Luis, M Martínez-Quiroz, A Ochoa-Terán, H Santacruz-Ortega, E Mendez-Valenzuela J Luminesce. 134 (2013) 729–738
Wu YS, Huang FF, Lin YW (2013) Fluorescent Detection of Lead in Environmental Water and Urine Samples Using Enzyme Mimics of Catechin-Synthesized Au Nanoparticles. ACS Appl Mater Interfaces 5:1503–1509
Prabhu J, Velmurugan K, Nandhakumar R (2015) Development of fluorescent lead II sensor based on an anthracene derived chalcone. Spectrochim Acta Part A 144:23–28
Zhan S, Wu Y, Luo Y, Liu L, He L, Xing H, Zhou P (2014) Label-free fluorescent sensor for lead ion detection based on lead(II)-stabilized G-quadruplex formation. Anal Biochem 462:19–25
X.Y. Wang, C.G. Niu, L.J. Guo, L-Yin Hu, S-Quan Wu, G-Ming Zeng, Fei Li J Fluoresce. 27 (2017) 643–649
Que EL, Domaille DW, Chang CJ (2008) Metals in Neurobiology: Probing Their Chemistry and Biology with Molecular Imaging. Chem Rev 108:1517–1549
Sarkar B (1999) Treatment of Wilson and Menkes Diseases. Chem Rev 99:2535–2544
Jang YK, Nam UC, Kwon HL, Hwang IH, Kim C (2013) A selective colorimetric and fluorescent chemosensor based-on naphthol for detection of Al3+ and Cu2+. Dyes Pigments 99:6–13
Zhang X, Kang M, Choi H, Jung JY, Swamy KMK, Kim S, Kim D, Kim J, Lee C, Yoon J (2014) Organic radical-induced Cu2+ selective sensing based on thiazolothiazole derivatives. Sensors Actuators B Chem 192:691–696
Yang X, Zhang W, Yi Z, Xu H, Wei J, Hao L (2017) Highly sensitive and selective fluorescent sensor for copper(ii) based on salicylaldehyde Schiff-base derivatives with aggregation induced emission and mechanoluminescence. New J Chem 41:11079–11088
Baslak C, Kursunlu AN (2018) A naked-eye fluorescent sensor for copper(ii) ions based on a naphthalene conjugate Bodipy dye. Photochem Photobiol Sci 17:1091–1097
Feng S, Gao Q, Gao X, Yin J, Inorg YJ Chem Commun 2019(102):51–56
Kumar J, Bhattacharyya PK, Das DK (2015) Spectrochim Acta 138:99
Ma Y, Li H, Peng S, Wang L (2012) Highly Selective and Sensitive Fluorescent Paper Sensor for Nitroaromatic Explosive Detection. Anal Chem 84:8415–8421
Sarma S, Bhowmik A, Sarma MJ, Banu S, Phukan P (2018) Condensation product of 2-hydroxy-1-napthaldehyde and 2-aminophenol: Selective fluorescent sensor for Al 3+ ion and fabrication of paper strip sensor for Al 3+ ion. Inorg Chim Acta 469:202–208
Jo S, Kim D, Son SH, Kim Y, Lee TS (2014) Conjugated Poly(fluorene-quinoxaline) for Fluorescence Imaging and Chemical Detection of Nerve Agents with Its Paper-Based Strip. ACS Appl Mater Interfaces 6:1330–1336
Su S, Ali MM, Filipe CDM, Li Y, Pelton R (2008) Microgel-Based Inks for Paper-Supported Biosensing Applications. Biomacromolecules 9:935–941
Kim Y, Jang G, Lee TS (2015) New Fluorescent Metal-Ion Detection Using a Paper-Based Sensor Strip Containing Tethered Rhodamine Carbon Nanodots. ACS Appl Mater Interfaces 7:15649–15657
Armstrong B, Tremblay C, Baris D, Theriault G (1994) Lung Cancer Mortality and Polynuclear Aromatic Hydrocarbons: A Case-Cohort Study of Aluminum Production Workers in Arvida, Quebec, Canada. Am J Epidemiol 139:250–262
Jain R, Gupta VK, Jadon N, Radhapyari K (2010) Voltammetric determination of cefixime in pharmaceuticals and biological fluids. Anal Biochem 407:79–88
Goyal RN, Gupta VK, Chatterjee S (2008) Electrochemical oxidation of 2′,3′-dideoxyadenosine at pyrolytic graphite electrode. Electrochim Acta 53:5354–5360
Cassella RJ, Magalhaes OIB, Couto MT, Lima ELS, Neves MAFS, Coutinho FMB (2005) Synthesis and application of a functionalized resin for flow injection/F AAS copper determination in waters. Talanta. 67:121–128
Mashhadizadeh MH, pesteh M, Talakesh M, Sheikhshoaie I, Ardakani MM, Karimi MA (2008) Solid phase extraction of copper (II) by sorption on octadecyl silica membrane disk modified with a new Schiff base and determination with atomic absorption spectrometry. Spectrochim Acta, Part B 63:885–888
Li YP, Liu XM, Zhang YH, Chang ZA (2013) A fluorescent and colorimetric sensor for Al3+ based on a dibenzo-18-crown-6 derivative. Inorg Chem Commun 33:6–9
Gupta VK, Singh AK, Kumawat LK (2014) Thiazole Schiff base turn-on fluorescent chemosensor for Al3+ ion. Sensors Actuators B Chem 195:98–108
Kaur K, Bhardwaj VK, Kaur N, Singh N (2012) Imine linked fluorescent chemosensor for Al3+ and resultant complex as a chemosensor for HSO4− anion. Inorg Chem Commun 18:79–82
Das DK, Kumar J, Bhowmik A, Bhattacharyya PK, Banu S (2017) Inorg Chim Acta 462:167–173
Kumar J, Sarma MJ, Phukan P, Das DK (2015) A new simple Schiff base fluorescence “on” sensor for Al3+and its living cell imaging. Dalton Trans 44:4576–4581
Fan L, Jiang X, Wang B, Yang Z (2014) 4-(8′-Hydroxyquinolin-7′-yl)methyleneimino-1-phenyl-2,3-dimethyl-5-pyzole as a fluorescent chemosensor for aluminum ion in acid aqueous medium. Sensors Actuators B Chem 205:249–254
Li-mei Liu, Zheng-yin Yang Inorg Chim Acta 469 (2018) 588–592, A new off-on fluorescent sensor for the detection of Al(III) based on a chromone-derived Schiff-base
Soufeena PP, Aravindakshan KK (2019) J Lumin 205:400–405
Sabah HH (2014) Der Pharma Chemica 6(2):38–41
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Accounts 120:215–241
Tomasi J, Persico M (1994) Molecular Interactions in Solution: An Overview of Methods Based on Continuous Distributions of the Solvent. Chem Rev 94:2027–2094
M. J. Frisch, et. al., Gaussian, Inc., Wallingford CT, 2016
Acknowledgements
The authors thank DST, New Delhi for financial grant vide MRP (EMR/2016/001745) and FIST to the department. IIT-Kanpur is thanked for HRMS spectra.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Das, D.K., Deka, S. & Guha, A.K. Schiff Base Derived from 4,4′-methylenedianiline and p-anisaldehyde: Colorimetric Sensor for Cu2+, Paper Strip Sensor for Al3+ and Fluorescent Sensor for Pb2+. J Fluoresc 29, 1467–1474 (2019). https://doi.org/10.1007/s10895-019-02443-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-019-02443-8