Abstract
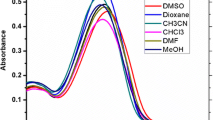
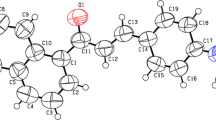
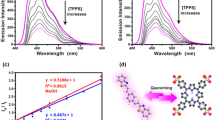
E,E-2,5-bis[2-(3-pyridyl)ethenyl]pyrazine (BPEP) has been prepared by aldol condensation between 2,5-dimethylpyrazine and pyridine-3-carboxaldehyde. It is characterized by IR, 1H NMR, and 13C NMR. The electronic absorption and emission properties of BPEP were studied in different solvents. BPEP displays a slight solvatochromic effect of the absorption and emission spectrum, indicating a small change in dipole moment of BPEP upon excitation. The dye solutions (1 × 10−4 M) in CHCl3, EtOH and dioxane give laser emission in blue region upon excitation by a 337.1 nm nitrogen pulse (λ = 337 nm). The tuning range, gain coefficient (α) and emission cross – section (σe) have been determined. Ground and excited states electronic geometric optimizations were performed using density functional theory (DFT) and time-dependent density functional theory (TD-DFT), respectively. A DFT natural bond analysis complemented the ICT. The simulated maximum absorption and emission wavelengths are in line the observed ones in trend, and are proportionally red-shifted with the increase of the solvent polarity. The stability, hardness and electrophilicity of BPEP in different solvents were correlated with the polarity of the elected solvents. BPEP dye displays fluorescence quenching by colloidal silver nanoparticles (AgNPs). The fluorescence data reveal that radiative and non-radiative energy transfer play a major role in the fluorescence quenching mechanism.









Similar content being viewed by others
References
A. C. Grimsdale, K. L Chan, R. E. Martin, P. G. Jokisz, A. B. Holmes, Chem. Rev 109 (2009) 897–1091.
Braga D, Horowitz G (2009) Adv Mater 21:1473–1486
Radford RJ, Chyan W, Lippard SJ (2013) Chem Sci 4:3080–3084
Facchetti A (2011) Chem Mater 23:733–758
Arias AC, MacKenzie JD, McCulloch I, Rivnay J, Salleo A (2010) Chem Rev 110:3–24
K.M. Rahulan, S. Balamurugan, K.S. Meena, G-Y. Yeap, C.C. Kanakam, Optics & Laser Technology 56 (2014) 142–145.
Singh H, Sindhu J, Khurana JM (2014) Sensors Actuators B 192:536–542
Grabowski ZR, Rotkiewicz K, Rettig W (2003) Chem Rev 103:3899–4302
Pham THN, Clarke RJ (2008) J Phys Chem B 112:6513
Huang Y, Cheng T, Li F, Huang C, Hou T, Yu A, Zhao X, Xu X (2002) J Phys Chem B 106:10020
Shaikh M, Mohanty J, Singh PK, Bhasikuttan AC, Rajule RN, Satam VS, Bendre SR, Kanetkar VR, Pal H (2010) J Phys Chem A 114:450
Kim HN, Guo Z, Zhu W, Yoon J, Tian H (2011) Chem Soc Rev 40:79–93
He GS, Tan LS, Zheng Q, Prasad PN (2008) Chem Rev 108:1245–1330
Li Z, Qin Q (2011) J Polym Chem 2:2723–2740
Castle RN (1962) Chemistry of heterocyclic compounds. John Wiley and Sons: New York 23
Hong B, Kang KA (2006) Biosens Bioelectron 21:1333
Ng MY, Liu WC (2009) Opt Express 17:5867
Daniel MC, Astruc D (2004) Chem Rev 104:293
Deng W, Goldys EM (2012) Langmuir 28:10152
Fu Y, Zhang J, Lakowicz JR (2007) J Fluoresc 17:811
Kalele S, Deshpande AC, Singh SB, Kulkarni SK (2008) Bull Mater Sci 31:541
Basheer NS, Kumar BR, Kurian A, George SD (2013) J Lumin 137:225–229
Pushpam S, Kottaisamy M, Ramakrishnan V (2013) Spectrochim Acta A Mol Biomol Spectrosc 114:272–276
Pannipara M, Asiri AM, Alamry KA, Arshad MN, El-daly SA (2014) J Fluoresc 24:1629–1638
Pannipara M, Asiri AM, Alamry KA, Arshad MN, El-Daly SA (2015) Spectrochim Acta A 136:1893–1902
Pannipara M, Asiri AM, Alamry KM, Salam IA, El-Daly SA (2015) J Lumin 157:163–171
Melhuish WH (1961) J Phys Chem 65:229–253
Coe BJ, Harris JA, Asselberghs I, Clays K, Olbrechts G, Persoons A, Hupp JT, Johnson RC, Coles SJ, Hursthous MB, Nakatani K (2002) Adv Funct Mater 12:110–116
Gordon P, Gregory P (1987) Organic chemistry in colour, Moskva, Chimia (in Russian)
Pushpam S, Kottaisamy M, Ramakrishnan Spectrochimica V (2013) Acta Part A 114:272–276
Lin-gun Liu, and W. A. Bassett, J. Appl. Phys., 44 (1973) 1475–1479.
Valeur B (2001) Molecular fluorescence: Principles and applications. Weinheim, Wiley-VCH Verlag
Rink M, Gusten H, Ache HJ (1986) Phys Chem 90:2661–2665
Nair LG (1982) Prog Quant Electrochem 7:153
Valverde-Aguilar G (2006) Opt Mater 28:1209–1215
Birks JB (1970) Photophysics of aromatic molecules. Wiley Interscience, London, Wiley, p. 88
Birks JB (1973) Organic molecular Photophysics. John Wiley and Sons, New York
Strickler SJ, Berg RA (1962) J Chem Phys 37:814–822
J. R. Lackowicz, Principle of Fluorescence Spectroscopy, Third Edition, 2006, Springer, New York Chapter 1, p. 10
Padilha LA, Webster S, Przhonska OV, Hu H, Peceli D, Enstly TR, Bondar MV, Gerasov AO, Kovtun YP, Shandura MP, Kachlkovski AD, Hagan DJ, Van Stryland EW, Phys J (2010) Chem A 114:6493–6501
Kumar GA, Unnikrishnan NV (1997) Solid State Commun 29:1049
Kumar GA, Unnikrishnan NV (2001) J. Photochem & Photobiol. A:Chem 144:107–117
El-Daly SA, Rahman MM, Alamry KA, Asiri AM (2014) J Lumin 148:303–306
El-Daly SA, Alamry KA, Asiri AM, Hussein MA (2012) J Lumin 132:2747–2752
Förster T (1996) In: Sinanoglu O (ed) Modern quantum Chemistry. Academic Press, New York
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox (2009). Gaussian 09, Revision A.02. Gaussian, Inc. Wallingford.
A. Frisch, R.D. Dennington II, T.A. Keith, J. Milliam. A. B. Nielsen, A. J. Holder, J. Hiscocks (2007). GaussView Reference, Version 5.0, Gaussian Inc. Pittsburgh.
Yanai T, Tew DP, Handy NCA (2004) New hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP) Chem. Phys Lett 393(1–3):51–57
Gross EKU, Kohn W (1990) Adv Quant Chem 21:255–291
Cances E, Mennucci B, Tomasi J (1997) J Chem Phys 107:3032–3041
Reed EA, Curtiss LA, Weinhold F (1988) Chem Rev 88:899
Wlodarczak G, Demaison J, Heineking N, Csaszar AG (1994) The Rotational Spectrum of propene: internal Rotation analysis and ab initio and experimental Centrifugal Distortion constants. J Mol Spectrosc 167:239–247
Penn RE (1978) Microwave Spectrum of 2-Propene-l-imine, CH,=CHCH = NH. J Mol Spectrosc 69(2):373–382
Silverstein RM, Bassler GC, Morrill TC (1991) Spectrometric Identification of organic compounds. John Willey, Chistester
Karabacak M, Cinar M (2012) Spectrochim Acta A 86:590–599
Luque, F.J., Lopez J.M., Orozco, M., Perspective on “Electrostatic interactions of a solute with a continuum. A direct utilization of ab initio molecular potentials for the provision of solvent effect”. Theor. Chem. Acc. (2000) 103:343–345.
Chattaraj PK, Maiti B (2003) HSAB principle applied to the time evolution of chemical reactions. J Am Chem Soc 125:2705–2710
Pearson RG (2005) Chemical hardness and density functional theory. J Chem Sci 117:369–377
Aurell MJ, Domingo LR, Perez P, Contreras R (2004) A theoretical study on the regioselectivity of 1,3-dipolar cycloadditions using DFT-based reactivity indexes. Tetrahedron 60:11503–11509
Reed EA, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint Chem. Rev 88(6):899–926
Reed EA, Weinhold F (1983) Natural bond orbital analysis of near-Hartree-Fock water dimer. J Chem Phys 78(6):4066–4073
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Al-Soliemy, A.M., Osman, O.I., Hussein, M.A. et al. Fluorescence, Photophysical Behaviour and DFT Investigation of E,E-2,5-bis[2-(3-pyridyl)ethenyl]pyrazine (BPEP). J Fluoresc 26, 1199–1209 (2016). https://doi.org/10.1007/s10895-016-1802-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1802-7