Abstract
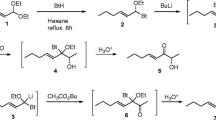
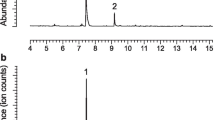
A novel trisubstituted tetrahydropyran was isolated and identified from the sex-specific volatiles produced by males of the cerambycid beetle Macropophora accentifer (Olivier), a serious pest of citrus and other fruit crops in South America. The compound was the major component in the headspace volatiles, and it was synthesized in racemic form. However, in field trials, the racemate was only weakly attractive to beetles of both sexes, suggesting that attraction might be inhibited by the presence of the “unnatural” enantiomer in the racemate. Alternatively, the male-produced volatiles contained a number of minor and trace components, including a compound tentatively identified as a homolog of the major component, as well as a number of unsaturated 8-carbon alcohols and aldehydes. Further work is required to conclusively identify and synthesize these minor components, to determine whether one or more of them are crucial components of the active pheromone blend for this species.





Similar content being viewed by others
Data Availability
Not applicable.
References
Allison JD, McKenney JL, Millar JG, McElfresh JS, Mitchell RF, Hanks LM (2012) Response of the woodborers Monochamus carolinensis and Monochamus titillator (Coleoptera: Cerambycidae) to known cerambycid pheromones in the presence and absence of the host plant volatile α-pinene. Env Entomol 41:1587–1596
Barry CSJ, Crosby SR, Harding JR, Hughes RA, King CD, Parker GD, Willis CL (2003) Stereoselective synthesis of 4-hydroxy-2,3,6-trisubstituted tetrahydropyrans. Org Lett 5:2429–2432
Bondar G (1913) Brocas das laranjeiras e outras auranciaceas. Bol Min Agric Ind Com, Rio de Janeiro 2:81–93 (in Portuguese)
Carrasco F (1978) Cerambícidos (Insecta, Coleoptera) sur-peruanos. Rev Peru Entomol 21:75–78 (in Spanish)
Chan KP, Loh TP (2005) Prins cyclizations in silyl additives with suppression of epimerization: versatile tool in the synthesis of the tetrahydropyran backbone of natural products. Org Lett 7:4491–4494
Chan KP, Ling YH, Chan JLT, Loh TP (2007) Stannane-free chemoselective hydrodehalogenation of 4-halotetrahydropyrans: scope and application to natural product synthesis. J Org Chem 72:2127–2132
Dede R, Michaelis L, Fuentes D, Yawer MA, Hussain I, Fischer C, Langer P (2007) Synthesis of 4-alkoxycarbonyl-butenolides by uncatalyzed one-pot cyclization of 1,3-bis(silyloxy)alk-1-enes with oxalyl chloride. Tetrahedron 63:12547–12561
Dobbs AP, Guesne SJJ, Parker RJ, Skidmore J, Stephenson RA, Hursthouse MBA (2010) Detailed investigation of the aza-Prins reaction. Org Biomol Chem 8:1064–1080
Duffy EAJ (1960) A monograph of the immature stages of Neotropical timber beetles (Cerambycidae). British Museum (Natural History), London
Eyre D, Haack RA (2017) Invasive cerambycid pests and biosecurity measures. In: Wang Q (ed) Cerambycidae of the world—biology and pest management. CRC Press, Boca Raton, pp 563–607
Fan J, Denux O, Courtin C, Bernard A, Javal M, Millar JG, Hanks LM, Roques A (2019) Multi-component blends for trapping native and exotic longhorn beetles at potential points-of-entry and in forests. J Pest Sci 92:281–297
Flaherty L, Gutowski JMG, Hughes C, Mayo P, Mokrzycki T, Pohl G, Silk P, Van Rooyen K, Sweeney J (2019) Pheromone-enhanced lure blends and multiple trap heights improve detection of bark and wood-boring beetles potentially moved in solid wood packaging. J Pest Sci 92:309–325
Francke W, Dettner K (2005) Chemical signalling in beetles. In: Schulz S (ed) The chemistry of pheromones and other semiochemicals II. Springer–Verlag, Berlin Heidelberg, pp 85–166
Garcia AH (1987) Ocorrência de escarabeídeos indicando a presença de larva de Macropophora accentifer (Olivier, 1975) em plantas cítricas. An Esc Agron Vet Univ Fed Goiás 17:37–42 (in Portuguese)
Garcia AH, Veloso VRS, Cunha MG (1993) Variedades de citros mais suscetíveis ao ataque de Macropophora accentifer (Olivier, 1795) Coleoptera, Cerambycidae. An Esc Agron Vet Univ Fed Goiás 23:187–197 (in Portuguese)
Haack RA (2017) Cerambycid pests in forests and urban trees. In: Wang Q (ed) Cerambycidae of the world: biology and pest management. CRC press. Taylor & Francis, Boca Raton, pp 351–407
Han X, Peh G, Floreancig PE (2013) Prins-type cyclization reactions in natural product synthesis. Eur J Org Chem 2013:1193–1208
Hanks LM, Millar JG (2016) Sex and aggregation-sex pheromones of cerambycid beetles: basic science and practical applications. J Chem Ecol 42:631–654
Hansen L, Xu T, Wickham J, Chen Y, Hao D, Hanks LM, Millar JG, Teale SA (2015) Identification of a male-produced pheromone component of the citrus longhorned beetle, Anoplophora chinensis. PloS ONE 10(8):e0134358
Kim WH, Jung JH, Lee E (2005) Feigrisolide C: structural revision and synthesis. J Org Chem 70:8190–8192
Machado LA, Raga A (1999) Eficiência de inseticidas químico e biológico sobre uma broca-do-tronco dos citrus. Rev Agric 74:229–234 (in Portuguese)
Macias-Samano JE, Wakarchuk D, Millar JG, Hanks LM (2012) 2-Undecyloxy-1-ethanol in combination with other semiochemicals attracts three Monochamus species (Coleoptera: Cerambycidae) in British Columbia, Canada. Can Entomol 144:764–768
Marumoto S, Jaber JJ, Vitale JP, Rychnovsky SD (2002) Synthesis of (−)-centrolobine by Prins cyclizations that avoid racemization. Org Lett 4:3919–3922
Meier LR, Zou Y, Millar JG, Mongold-Diers J, Hanks LM (2016) Synergism between enantiomers creates species-specific pheromone blends and minimizes cross-attraction for two species of cerambycid beetles. J Chem Ecol 42:1181–1192
Meier LR, Zou Y, Millar JG, Mongold-Diers J, Hanks LM (2020) Pheromone composition and chemical ecology of six species of cerambycid beetles in the subfamily Lamiinae. J Chem Ecol 46:30–39
Meurisse N, Rassati D, Hurley BP, Brockerhoff EG, Haack RA (2019) Common pathways by which non-native forest insects move internationally and domestically. J Pest Sci 92:13–27
Millar JG, Hanks LM (2017) Chemical ecology of cerambycids. In: Wang Q (ed) Cerambycidae of the world: biology and pest management. CRC press. Taylor & Francis, Boca Raton, pp 167–202
Millar JG, Mitchell RF, Meier LR, Johnson TD, Mongold-Diers JA, Hanks LM (2017) (2E,6Z,9Z)-2,6,9-Pentadecatrienal as a male-produced aggregation-sex pheromone of the cerambycid beetle Elaphidion mucronatum. J Chem Ecol 43:1056–1065
Mitchell RF, Reagel PF, Wong JCH, Meier LR, Silva WD, Mongold-Diers J, Millar JG, Hanks LM (2015) Cerambycid beetle species with similar pheromones are segregated by phenology and minor pheromone components. J Chem Ecol 41:431–444
Monné MA (2021) Catalogue of the Cerambycidae (Coleoptera) of the Neotropical region. Part II. Subfamily Lamiinae https://cerambycids.com/default.asp?action=show_catalog. Accessed 25 January 2022
Monné ML, Monné MA, Wang G (2017) General morphology, classification, and biology of Cerambycidae. In: Wang Q (ed) Cerambycidae of the world: biology and pest management. CRC press. Taylor & Francis, Boca Raton, pp 1–70
Moreira C (1912) Insectos nocivos às laranjeiras e meios para destrui-los. Alm Ag Bras, São Paulo 1:129–134 (in Portuguese)
Motodate S, Kobayashi T, Fujii M, Mochida T, Kusakabe T, Katoh S, Akita H, Kato K (2010) Synthesis of β-methoxyacrylate natural products based on box-Pdii-catalyzed intermolecular methoxycarbonylation of alkynoles. Chem Asian J 5:2221–2230
Olier C, Kaafarani M, Gastaldi S, Bertrand M (2010) Synthesis of tetrahydropyrans and related heterocycles via Prins cyclization; extension to aza-Prins cyclization. Tetrahedron 66:413–445
Santos-Silva A, Botero JP, Nascimento FE, de Lima Nascimento FE, Silva WD (2020) A new synonym and seventeen new distributional records in south American Cerambycidae (Coleoptera), with notes on Chlorethe scabrosa Zajciw, 1963. Pap Avulsos Zool 60:e20206010
SAS Institute (2011) SAS/STAT v. 9.3 User’s Guide. In: SAS Institute Inc, Cary NC
Schallenberger E (1994) “Fatores que predispoem as plantas cítricas ao ataque de coleobrocas” Master thesis, Universidade Estadual Paulista “Júlio De Mesquita Filho” (in Portuguese)
Silva WD, Millar JG, Hanks LM, Bento JMS (2016) (6E,8Z)-6,8-Pentadecadienal, a novel attractant pheromone produced by males of the cerambycid beetles Chlorida festiva and Chlorida costata. J Chem Ecol 42:1082–1085
Silva WD, Bento JMS, Hanks LM, Millar JG (2018) (Z)-7-Hexadecene is an aggregation-sex pheromone produced by males of the south American cerambycid beetle Susuacanga octoguttata. J Chem Ecol 44:1115–1119
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W. H.Freeman, New York
Tavakilian G, Chevillotte H (2022) Titan database about longhorns or timber-beetles (Cerambycidae) http://titan.gbif.fr/accueil_uk.html. Accessed 25 January 2022
Teale SA, Wickham JD, Zhang F, Su J, Chen Y, Xiao W, Hanks LM, Millar JG (2011) A male-produced aggregation pheromone of Monochamus alternatus (Coleoptera: Cerambycidae), a major vector of pine wood nematode. J Econ Ent 104:1592–1598
Wang Q (2017) Cerambycid pests in agricultural and horticultural crops. In: Wang Q (ed) Cerambycidae of the world: biology and pest management. CRC press. Taylor & Francis, Boca Raton, pp 409–562
Widmer U (1987) A convenient benzylation procedure for β-hydroxy esters. Synthesis 1987:568–570
Xu T, Yasui H, Teale SA, Fujiwara-Tsujii N, Wickham JD, Fukaya M, Hansen L, Kiriyama S, Hao D, Nakano A, Zhang L, Watanabe T, Tokoro M, Millar JG (2017) Identification of a male-produced sex-aggregation pheromone for a highly invasive cerambycid beetle, Aromia bungii. Sci Rep 7:7330
Yadav JS, Reddy BVS, Kumar GN, Swamy T (2007) Iodine as a versatile reagent for the Prins-cyclization: an expeditious synthesis of 4-iodotetrahydropyran derivatives. Tetrahedron Lett 48:2205–2208
Yang XF, Mague JT, Li CJ (2001) Diastereoselective synthesis of polysubstituted tetrahydropyrans and thiacyclohexanes via indium trichloride mediated cyclization. J Org Chem 66:739–747
Zou Y, Millar JG, Blackwood JS, Van Duzor R, Hanks LM, Mongold-Diers JA, Wong JC, Ray AM (2015) (2S,4E)-2-Hydroxy-4-octen-3-one, a male-produced attractant pheromone of the cerambycid beetle Tylonotus bimaculatus. J Chem Ecol 41:670–677
Acknowledgements
We thank Citrosuco S/A Agroindústria for allowing access to the experimental sites at Rio Pardo Farm and for providing logistical support to this research. A special thank you to Germano Pires Galhardo and Denis Rogério Marin for assisting with the field experiments and for providing the infested citrus trunks. We also thank Antonio Santos-Silva (Museum of Zoology, USP) for identifying the cerambycid species. Field collections of the study species in Brazil were conducted under SISBIO permit #46395 from the Brazilian Ministry of the Environment. This work was registered with the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen, Brazil) under #AE3897B.
Code Availability
Not applicable.
Funding
This work was supported by Instituto Nacional de Ciencia e Tecnologia de Semioquímicos na Agricultura (Fundação de Amparo à Pesquisa do Estado de São Paulo and Conselho Nacional de Desenvolvimento Científico e Tecnológico, grant #s 2014/50871-0 and 465511/2014-7 to JMB) and the United States Department of Agriculture – Animal and Plant Health Inspection Service (grant #s 15, 16, 17, 18, 19, 20, and 21-8130-1422-CA to JGM and LMH, and grant # 21-8130-0909-CA to WDS).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. WDS collected insects, prepared extracts, carried out initial analyses, and carried out field trials. JGM identified the compound and carried out the first synthesis. YZ carried out the synthesis of the pure diastereomer. WDS and LMH carried out the data analyses, and WDS, YZ, and JGM drafted the manuscript, which was then edited by all authors. Funding was obtained by JGM, LMH, and JMB.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Additional information
Dedicated to the memory of Professor Kenji Mori
Supplementary Information
Supplementary online information includes the synthetic procedures for the nonstereoselective synthesis of the major insect-produced compound, the proton and gCOSY NMR spectra of the isolated compound in CD2Cl2, the GC-EAD analyses of synthetic THP, the proton spectra of the synthesized THP compound in both CD2Cl2 and C6D6 solvents, the EI mass spectra of minor components A–G, I, and K in the volatiles from male beetles, and analyses of insect-produced and synthetic racemic THP on a chiral stationary phase GC column..
ESM 1
(PDF 817 kb)
Rights and permissions
About this article
Cite this article
Silva, W.D., Zou, Y., Hanks, L.M. et al. A Novel Trisubstituted Tetrahydropyran as a Possible Pheromone Component for the South American Cerambycid Beetle Macropophora accentifer. J Chem Ecol 48, 569–582 (2022). https://doi.org/10.1007/s10886-022-01362-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-022-01362-6