Abstract
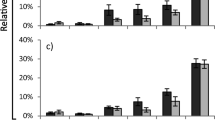
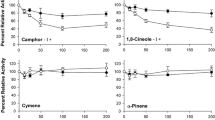
The privet tree, Ligustrum obtusifolium (Oleaceae), defends its leaves against insects with a strong lysine-decreasing activity that make proteins non-nutritive. This is caused by oleuropein, an iridoid glycoside. We previously found that some privet-specialist caterpillars adapt by secreting glycine in the digestive juice as a neutralizer that prevents the loss of lysine. Here, we extended the survey into 42 lepidopteran and hymenopteran species. The average concentration of glycine in digestive juice for 11 privet-feeding species (40.396 mM) was higher than that for 32 non-privet-feeding species (2.198 mM). The glycine concentrations exceeded 10 mM in 7 out of 11 privet-feeding species. In Macrophya timida (Hymenoptera), it reached 164.8 mM. Three out of the four remaining privet-feeding species had other amino acids instead. Larvae of a privet-specialist butterfly, Artopoetes pryeri (Lycaenidae), had a high concentration (60.812 mM) of GABA. In two other specialists, β-alanine was found. GABA, β-alanine, and glycine as well as alanine, amines, and ammonium ion inhibited the lysine decrease, indicating that amino residues are responsible for the inhibition. However, the three amino acids found in the specialists were far more effective (20 mM showed 80% inhibition) than the rest (>140 mM was required for 80% inhibition). Our results show a clear and rare case of the apparent convergent evolution of herbivores’ molecular adaptations of feeding on a plant with a chemical defense in a manner that minimizes the cost of adaptation. The novel role of GABA in plant-herbivore interactions shown here is probably the first reported non-neuronal role of animal-derived GABA.



Similar content being viewed by others
References
Agrawal, A. A. 2000. Host-range evolution: adaptation and trade-offs in fitness of mites on alternative hosts. Ecology 81:500–508.
Berenbaum, M. 1978. Toxicity of a furanocoumarin to armyworms: a case of biosynthetic escape from insect herbivores. Science 201:532–533.
Berenbaum, M. R. 1991. Coumarins, pp. 221–249 in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores 2nd Ed., Vol. I. The Chemical Participants. Academic Press, San Diego, California.
Bianco, A. D., Muzzalupo, I., Piperno, A., Romeo, G., and Uccella, N. 1999a. Bioactive derivatives of oleuropein from olive fruits. J. Agric. Food Chem. 47:3531–3534.
Bianco, A. D., Piperno, A., Romeo, G., and Uccella, N. 1999b. NMR experiments of oleuropein biomimetic hydrolysis.J. Agric Food Chem. 47:3665–3668.
Bowers M. D. 1991. Iridoid glycosides, pp. 297–326, in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores 2nd Ed., Vol. I. The Chemical Participants. Academic Press, San Diego, California.
Broadway, R. M. 1995. Are insects resistant to plant proteinase inhibitors? J. Insect Physiol. 41:107–116.
Chen, H., Wilkerson, C. G., Kuchar, J. A., Phinney, B. S., and Howe G. A. 2005. Jasmonate- inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc. Natl. Acad. Sci. U.S.A. 102:19237–19242.
Felton, G. W. and Duffey, S. S. 1991. Protective action of midgut catalase in lepidpteran larvae against oxidative plant defenses. J. Chem. Ecol. 17:1715–1732.
Felton, G. W., Donato, K., Del Vecchio, R. J., and Duffey, S. S. 1989. Activation of plant foliar oxidases by insect feeding reduces nutritive quality of foliage for noctuid herbivores. J. Chem. Ecol. 15:2667–2694.
Felton G.W. & Gatehouse J.A. 1996: Antinutritive plant defence mechanisms. pp. 373–416 in Lehane M.J. & Billingsley P.F. (eds): Biology of the Insect Midgut. The University Press, Cambridge.
Futuyma, D. J. and M. C. Keese, M. C. 1992. Evolution and coevolution of plants and phytophagous arthropods, pp. 439-475, in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores 2nd Ed., Vol. I. Ecological and Evolutionary Processes. Academic Press, San Diego, California
Gould, F. 1988. Genetics of pairwise and multispecies plant-herbivore coevolution, pp. 13–55, in K. C. Spencer (ed.). Chemical Mediation of Coevolution. Academic Press, San Diego, California.
Jung, V. and Ponert, G. 2001. Rapid wound-activated transformation of the green algal defensive metabolite caulerpenyne. Tetrahederon 57: 7169–7172.
Kawahara, J., Ohmori, T., Ohkubo, T., Hattori, S., and Kawamura, M. 1992. The structure of glutaraldehyde in aqueous solution determined by ultra violet absorption and light scattering. Anal. Biochem. 201:94–98.
Konno, K., Hirayama, C., and Shinbo, H. 1996. Unusually high concentration of free glycine in the midgut content of the silkworm, Bombyx mori, and other lepidopteran larvae. Comp. Biochem. Physiol. A 115:229–235.
Konno, K., Hirayama, C., and Shinbo, H. 1997. Glycine in digestive juice: a strategy of herbivorous insects against chemical defense of host plants. J. Insect Physiol. 43:217–224.
Konno, K., Yasui, H., Hirayama, C., and Shinbo, H. 1998. Glycine protects against strong protein-denaturing activity of oleuropein, a phenolic compound in privet leaves. J. Chem. Ecol. 24:735–751.
Konno, K., Hirayama, C., Yasui, H., and Nakamura, M. 1999. Enzymatic actyivation of oleuropein: a protein crosslinker used as a chemical defense in the privet tree. Proc. Natl. Acad. Sci. U.S.A. 96:9159–9164.
Konno, K., Okada, S., and Hirayama, C. 2001. Selective secretion of free glycine, a neutralizer against a plant defense chemical, in the digestive juice of the privet moth larvae. J. Insect Physiol. 47:1451–1457.
Konno, K., Hirayama, C., Shinbo, H., and Nakamura M. 2009. Glycine addition improves feeding performance of non-specialist herbivores on the privet, Ligustrum obtusifolium: In vivo evidence for the physiological impacts of anti-nutritive plant defense with iridoid and insect adaptation with glycine. Appl. Entomol. Zool. 44:595–601.
Lambers, H., Chapin III, F. S., and Pons, T. L. 1998. Plant Physiological Ecology. Springer-Verlag, New York.
Lawrence, P. K. and Koundal, K. R. 2002. Plant protease inhibitors in control of phytophagous insects. Electron. J. Biotechnol. 5:93–104.
Richards, F. M., and Knowles J. R. 1968. Glutaraldehyde as a protein cross-linking reagent. J. Mol. Biol. 37:231–233.
Shimada, T. 2006. Salivary proteins as a defense against dietary tannins. J. Chem. Ecol. 32:1149–1163.
Touyama, R., Takeda, Y., Inoue, K., Kawamura, I., Yatsuzuka, M., Ikumoto, T., Shingu, T., Yokoi, T., and Inoue, H. 1994. Studies on the blue pigments produced from genipin and methylamine. I. Structures of the brownish-red pigments, intermediates leading to blue pigments.Chem. Pharm. Bull. 42:668–673.
Waagepetersen, H. S., Sonnewalt, U., and Schausboe, A. 1999. The GABA paradox: multiple roles as metabolite, neurotransmitter, and neurodifferentiative agent. J. Neurochem. 73:1335–1342.
Weissflog, J., Adolph, S., Wiesemeier, T., and Pohnert, G. 2008. Reduction of herbivory through wound-activated protein cross-linking by the invasive macroalga, Caulerpa taxifolia. ChemBioChem 9:29–32.
Zhu-Salzman, K., Luthe, D. S., and Felton, G. W. 2008. Arthropod-inducible proteins: Broad spectrum defenses against multiple herbivores. Plant Physiol. 146:852–858.
Acknowledgements
We thank Prof. Keiichi Honda in Hiroshima University for invaluable suggestions, and Mrs. Mayumi Hazeyama for rearing insects. This research was supported by the Enhancement of Center of Excellence, Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), and by the Pioneer Research Project Fund from the Ministry of Agriculture, Forestry and Fisheries of Japan (MAFF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Konno, K., Hirayama, C., Yasui, H. et al. GABA, β-Alanine and Glycine in the Digestive Juice of Privet-Specialist Insects: Convergent Adaptive Traits Against Plant Iridoids. J Chem Ecol 36, 983–991 (2010). https://doi.org/10.1007/s10886-010-9842-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9842-y