Abstract
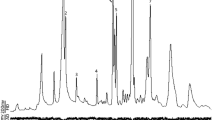
Many polyphagous insect species are important economic pests on one or more of their crop hosts. For most important insect pests, the common crop hosts are well-known, but knowledge of weedy and unmanaged hosts is limited. Furthermore, the relative contribution of different hosts to local and regional populations has rarely been ascertained because this requires having some way to determine which plant hosts are the source of the adult moths observed ovipositing in a crop field at a given place and time. One way of determining the larval host of polyphagous pest species is to analyze for several plant-derived chemicals that are each specific to a different small set of related plant species and are preserved in detectable amounts in adult moths. In this paper, we describe novel methods for analyzing adults of the polyphagous lepidopteran, the tobacco budworm (TBW) Heliothis virescens (F.), for plant secondary metabolites, specifically cotinine and gossypol, which are diagnostic for larval feeding on tobacco and cotton, respectively. Cotinine was extracted from individual TBW moths with acetic acid and methanol, then concentrated and analyzed directly by gas chromatography/mass spectrometry (GC/MS). The same moths then were analyzed for bound gossypol by creating a Schiff’s base that used aniline, and the resulting dianilino–gossypol complex was quantified using high pressure chromatography coupled with a triple quadrupole mass spectrometer (MS) as the detector. Based on analysis of standards, the detection limit for the cotinine was less than 1.5 ppb by dry weight. Comparable standards were not available for the gossypol derivative so a quantitative limit of detection could not be calculated. When TBW moths reared on known hosts were analyzed for gossypol and/or cotinine, all of the moths reared on tobacco or cotton were correctly identified, although some false positives were recorded with the gossypol method. Analysis of TBW moths of various ages and at various lengths of time after death determined that a significant gossypol signal was detectable in all moths reared on cotton. TBW moths collected from the vicinity of cotton fields in July and August in North Carolina also were analyzed. A much larger portion of the moths were derived from tobacco (6.7–46.4%) than from cotton (0–3.6%) in both months. Thus, these methods can be reliably used to estimate the proportion of TBW derived from noncotton host plants in populations trapped around Bt cotton fields, thereby providing insight into the risk of TBW evolving resistance to Bt cotton.







Similar content being viewed by others
References
Benson, C. G., Wyllie, S. G., Leach, D. N., and Fitt, G. P. 2001. Improved method for rapid determination of terpenoid aldehydes in cotton. J. Agric. Food Chem. 49:2181–2184.
Dabrowski, K., Lee, K. J., Rinchard, J., Ciereszko, A., Blom, J. H., and Ottobre, J. S. 2001. Gossypol isomers bind specifically to blood plasma proteins and spermatozoa of rainbow trout fed diets containing cottonseed meal. Biochim. Biophys. Acta 1525:37–42.
Deppermann, K. L. 2005. U.S. Patent number 6,880,771.
Fitt, G. P. 1989. The ecology of Heliothis species in relation to agroecosystems. Annu. Rev. Entomol. 34:17–52.
Gould, F., Blair, N., Reid, M., Rennie, T. L., Lopez, J., and Micinski, S. 2002. Bacillus thuringiensis-toxin resistance management: Stable isotope assessment of alternate host use by Helicoverpa zea. Proc. Natl. Acad. Sci. U S A 99:16581–16586.
Kim, H. L., Calhoun, M. C., and Stipanovic, R. D. 1996. Accumulation of gossypol enantiomers in ovine tissues. Comp. Biochem. Physiol. 113B:417–420.
Kim, I., Darwin, W. D., and Huestis, M. A. 2005. Simultaneous determination of nicotine, cotinine, norcotinine, and trans-3′-hydroxcotinine in human oral fluid using solid phase extraction and gas chromatography-mass spectrometry. J. Chromatography B 814:233–240.
Pons, W. A. Jr., Pittman, R. A., and Hoffpauir, C. L. 1958. 3 Amino-1-propanol as a complexing agent in the determination of total gossypol. J. Am. Oil Chem. Soc. 35:93–97.
Ravi, K. C., Mohan, K. S., Manjunath, T. M., Head, G., Patil, B. V., Greba, D. P. A., Premalatha, K., Peter, J., and Rao, N. G. V. 2005. Relative abundance of Helicoverpa armigera (Lepidoptera: Noctuidae) on different host crops in India and the role of these crops as natural refuge for Bacillus thuringiensis cotton. Environ. Entomol. 34:59–69.
Rojas, M. G., Stipanovic, R. D., Williams, H. J., and Vinson, S. B. 1992. Metabolism of gossypol by Heliothis virescens (F.) (Lepidotera: Noctuidae). Environ. Entomol. 21:518–526.
SAS Institute. 2001. SAS, Version 8.2. SAS Institute, Cary, NC.
Smith, F. N. 1967. Determination of gossypol in leaves and flower buds of Gossypium. J. Am. Oil Chem. Soc. 44:267–269.
Stipanovic, R. D., Williams, H. J., Smith, L. A., and Vinson, S. B. 1985. The hormetic effect of gossypol on the growth of Heliothis virescens larvae, pp. 392–393, in J. M. Brown and T. C. Nelson (eds.). Proceedings—Beltwide Cotton Conferences. NCC, Memphis, TN.
Wu, K., Guo, Y., and Gao, S. 2002. Evaluation of the natural refuge function for Helicoverpa armigera (Lepidoptera: Noctuidae) within Bacillus thuringiensis transgenic cotton growing areas in North China. J. Econ. Entomol. 95:832–837.
Acknowledgments
We thank Ryan Jackson (USDA-ARS, Stoneville, MS) for providing the TBW moths reared on known hosts and for coding samples for the blind experiment on gossypol signal stability. James Jennings, Melinda McCann, and Eric Sachs (Monsanto Company) provided helpful comments on the manuscript. Sharon Alexander and Mary Anderson (Monsanto Company) contributed tireless technical assistance with the gossypol assays.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orth, R.G., Head, G. & Mierkowski, M. Determining Larval Host Plant Use by a Polyphagous Lepidopteran Through Analysis of Adult Moths for Plant Secondary Metabolites. J Chem Ecol 33, 1131–1148 (2007). https://doi.org/10.1007/s10886-007-9284-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9284-3