Abstract
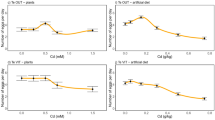
Plants that contain elevated foliar metal concentrations can be categorized as accumulators or, if the accumulation is extreme, hyperaccumulators. The defense hypothesis suggests that these plants may be defended against folivore attack, and recent research has indicated that metal concentrations at or below the accumulator range may be defensively effective. This experiment explored the toxicity of four metals hyperaccumulated by plants (Cd, Ni, Pb, and Zn) and asked if combinations of metals, or metals and organic chemicals, might broaden the defensive effectiveness of metals. Metals were used alone and in certain metal + metal (Zn plus Ni, Pb, or Cd) and metal + organic defensive chemical (Ni plus tannic acid, atropine, or nicotine) combinations. Artificial diet amended with these treatments was fed to larvae of the crucifer specialist herbivore Plutella xylostella. Combinations of metals and metals + organic chemicals significantly decreased survival and pupation rates, compared to single treatments, for at least some concentrations in every experiment. Effects of combinations were additive rather than synergistic or antagonistic. Because Zn enhanced the toxicity of other metals and Ni enhanced the toxicity of organic defensive chemicals, our findings suggest that the defensive effects of metals are more widespread among plants than previously believed. They also support the hypothesis that herbivore defense may have led to the evolution of metal hyperaccumulation by increasing the preexisting defensive effects of metals at accumulator levels in plants.






Similar content being viewed by others
References
Agrawal, A. A. 2000. Benefits and costs of induced plant defense for Lepidium virginicum (Brassicaceae). Ecology 81:1804–1813.
Agrawal, A. A. 2005. Future directions in the study of induced plant responses to herbivory. Entomol. Exp. Appl. 115:97–105.
Baker, A. J. M. 1981. Accumulators and excluders—strategies in the response of plants to heavy metals. J. Plant Nutr. 3:634–654.
Baker, A. J. M. and Brooks, R. R. 1989. Terrestrial plants which hyperaccumulate metallic elements—a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126.
Baker, A. J. M. and Brooks, R. R. 1994. The possibility of in situ heavy metal decontamination of polluted soils using crops of metal-accumulating plants. Resour. Conserv. Recycl. 11:41–49.
Baker, A. J. M., MCGrath, S. P., Reeves, R. D., and Smith, J. A. C. 2000. Metal hyperaccumulator plants: A review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils, pp. 85–108, in N. Terry and G. S. Bañuelos (eds.). Phytoremediation of Contaminated Soil and Water. Lewis Publishers, Boca Raton.
Behmer, S. T., Simpson, S. J., and Raubenheimer, D. 2002. Herbivore foraging in chemically heterogeneous environments: Nutrients and secondary metabolites. Ecology 83:2489–2501.
Borhidi, A. 2001. Phylogenetic trends in Ni-accumulating plants. S. Afr. J. Sci. 97:544–547.
Boyd, R. S. 1998. Hyperaccumulation as a plant defensive strategy, pp. 181–201, in R. R. Brooks (ed.). Plants that Hyperaccumulate Heavy Metals. CAB International, Oxford, UK.
Boyd, R. S. 2004. Ecology of metal hyperaccumulation. New Phytol. 162:563–567.
Boyd, R. S. and Martens, S. N. 1994. Nickel hyperaccumulated by Thlaspi montanum var. montanum is acutely toxic to an insect herbivore. Oikos 70:21–25.
Boyd, R. S. and Moar, W. J. 1999. The defensive function of Ni in plants: Response of the polyphagous herbivore Spodoptera exigua (Lepidoptera: Noctuidae) to hyperaccumulator and accumulator species of Streptanthus (Brassicaceae). Oecologia 118:218–224.
Boyd, R. S. and Shaw, J. J. 2004. Response of Xanthomonas campestris to metals: Implications for hyperaccumulation as a pathogen defense, pp. 279–282, in R. S. Boyd, A. J. M. Baker, and J. Proctor (eds.). Ultramafic Rocks: Their Soils, Vegetation and Fauna. Science Reviews Ltd., St Albans, Herts, UK.
Brooks, R. R. 1987. Serpentine and Its Vegetation. Dioscorides Press, Portland.
Brooks, R. R., Lee, J., Reeves, R. D., and Jaffré , T. 1977. Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J. Geochem. Explor. 7:49–57.
Carpenter, J. E. and Bloem, S. 2002. Interaction between insect strain and artificial diet in diamondback moth development and reproduction. Entomol. Exp. Appl. 102:283–294.
Clausen, T. P., Reichardt, P. B., and Bryant, J. P. 1992. Condensed tannins in plant defense: A perspective on classical theories, pp. 639–652, in R. W. Hemingway and P. E. Laks (eds.). Plant Polyphenols: Synthesis, Properties, Significance. Plenum Press, New York.
Coleman, C. M., Boyd, R. S., and Eubanks, M. D. 2005. Extending the elemental defense hypothesis: Dietary metal concentrations below hyperaccumulator levels could harm herbivores. J. Chem. Ecol. 31:1669–1681.
Dyer, L. A., Dodson, C. D., Stireman, J. O., Tobler, M. A., Smilanich, A. M., Fincher, R. M., and Letourneau, D. K. 2003. Synergistic effects of three piper amides on generalist and specialist herbivores. J. Chem. Ecol. 29:2499–2514.
Escarré , J., Lefèbvre, C., Gruber, W., Leblanc, M., Lepart, J., Rivière, Y., and Delay, B. 2000. Zinc and cadmium hyperaccumulation by Thlaspi caerulescens from metalliferous and nonmetalliferous sites in the Mediterranean area: Implications for phytoremediation. New Phytol. 145:429–437.
Feeny, P. 1976. Plant apparency and chemical defense. Recent Adv. Phytochem. 10:1–40.
Gatehouse, J. A. 2002. Plant resistance towards insect herbivores: A dynamic interaction. New Phytol. 156:145–169.
Gómez, D., Azorón, J., Bastida, J., Viladomat, F., and Codina, C. 2003. Seasonal and spatial variations of alkaloids in Merendera montana in relation to chemical defense and phenology. J. Chem. Ecol. 29:1117–1126.
Hanson, B. R., Lindblom, S. D., Loeffler, M. L., and Pilon-smits, E. A. H. 2004. Selenium protects plants from phloem-feeding aphids due to both deterrence and toxicity. New Phytol. 162:655–662.
Harvey, C. 2002. The effects of habitat complexity on biological control of herbivores and natural enemy interactions. MS Thesis, Auburn University, Alabama.
Hay, M. E. 1996. Defensive synergisms? Reply to Pennings. Ecology 77:1950–1952.
Hay, M. E., Kappel, Q. E., and Fenical, W. 1994. Synergisms in plant defenses against herbivores: Interactions of chemistry, calcification, and plant quality. Ecology 75:1714–1726.
Huitson, S. and Macnair, M. R. 2003. Does zinc protect the zinc hyperaccumulator Arabidopsis halleri from herbivory by snails? New Phytol. 159:453–459.
Iturralde, R. B. 2004. Notes on tropical American nickel accumulating plants, pp. 255–258, in R. S. Boyd, A. J. M. Baker, and J. Proctor (eds.). Ultramafic Rocks: Their Soils, Vegetation and Fauna. Science Reviews Ltd., St Albans, Herts, UK.
Jhee, E. M., Dandridge, K. L., and Christy, JR., A. M., and Pollard, A. J. 1999. Selective herbivory on low-zinc phenotypes of the hyperaccumulator Thlaspi caerulescens (Brassicaceae). Chemoecology 9:93–95.
Jhee, E. M., Boyd, R. S., Eubanks, M. D., and Davis, M. A. 2005. Nickel hyperaccumulation by Streptanthus polygaloides protects against the folivore Plutella xylostella (Lepidoptera: Plutellidae). Plant Ecol. DOI 10.1007/s11258-005-9009-z.
Louda, S. and Mole, S. 1991. Glucosinolates: Chemistry and ecology, pp. 123–164, in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores: Their Interactions with Secondary Plant Metabolites. Academic Press, Inc., San Diego.
Macnair, M. R. 2003. The hyperaccumulation of metals by plants. Adv. Bot. Res. 40:63–105.
Martens, S. N. and Boyd, R. S. 1994. The ecological significance of nickel hyperaccumulation: A plant chemical defense. Oecologia 98:379–384.
Mcnaughton, S. J. and Tarrants, J. L. 1983. Grass leaf silicification: Natural selection for an inducible defense against herbivores. Proc. Natl. Acad. Sci. U.S.A. 80:790–791.
Meerts, P. and van Isacker, N. 1997. Heavy metal tolerance and accumulation in metallicolous and non-metallicolous populations of Thlaspi caerulescens from continental Europe. Plant Ecol. 133:221–231.
Mesjasz-przybylowicz, J. and Przybylowicz, W. J. 2001. Phytophagous insects associated with the Ni-hyperaccumulating plant Berkheya coddii (Asteraceae) in Mpumalanga, South Africa. S. Afr. J. Sci. 97:596–598.
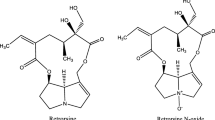
Muller, J. L. 1998. Love potions and the ointment of witches: Historical aspects of nightshade alkaloids. J. Toxicol., Clin. Toxicol. 36:617–627.
Nelson, A. C. and Kursar, T. A. 1999. Interactions among plant defense compounds: A method for analysis. Chemoecology 9:81–92.
Noret, N., Meerts, P., Tolrà, R., Poschenrieder, C., Barceló, J., and Escarré, J. 2005. Palatability of Thlaspi caerulescens for snails: influence of zinc and glucosinolates. New Phytol. 165:763–772.
Pennings, S. C. 1996. Testing for synergisms between chemical and mineral defenses—a comment. Ecology 77:1948–1950.
Pollard, A. J. and Baker, A. J. M. 1997. Deterrence of herbivory by zinc hyperaccumulation in Thlaspi caerulescens (Brassicaceae). New Phytol. 135:655–658.
Reeves, R. D. and Baker, A. J. M. 1984. Studies on metal uptake by plants from serpentine and non- serpentine populations of Thlaspi goesingense Halacsy (Cruciferae). New Phytol. 98:191–204.
Reeves, R. D. and Baker, A. J. M. 2000. Metal-accumulating plants, pp. 193–229, in I. Raskin and B. D. Ensley (eds.). Phytoremediation of Toxic Metals. Wiley, New York.
Reeves, R. D. and Brooks, R. R. 1983. Hyperaccumulation of lead and zinc by two metallophytes from mining areas of Central Europe. Environ. Pollut. A 31:277–285.
Reeves, R. D., Brooks, R. R., and Macfarlane, R. M. 1981. Nickel uptake by Californian Streptanthus and Caulanthus with particular reference to the hyperaccumulator S. polygaloides Gray (Brassicaceae). Am. J. Bot. 68:708–712.
Rhoades, D. F. 1979. Evolution of plant chemical defense against herbivores, pp. 1–55, in G.A. Rosenthal and D.H. Janzen (eds.). Herbivores: Their Interaction with Secondary Plant Metabolites. Academic Press, New York.
Rhoades, D. F. and Cates, R. G. 1976. Toward a general theory of plant antiherbivore chemistry. Recent Adv. Phytochem. 10:168–213.
Salama, H. S., Foda, M. S., Zaki, F. N., and Moawad, S. 1984. Potency of combinations of Bacillus thuringiensis and chemical insecticides on Spodoptera littoralis (Lepidoptera: Noctuidae). J. Econ. Entomol. 77:885–890.
SAS Institute. 2005. JMP in 5.1. Thomson–Brooks/Cole, Belmont.
Schultz, J. C. 1988. Tannin–insect interactions, pp. 621–638, in R.W. Hemingway and J.J. Karchesy (eds.). Chemistry and Significance of Condensed Tannins. Plenum Press, New York.
Shelton, A. M. and Collins, H. L. 2000. Techniques for rearing Plutella xylostella at N.Y.S. Agricultural Experiment Station Geneva, New York. Shelton Lab, Cornell University's New York State Agricultural Research Station, Geneva.
Talekar, N. S. and Shelton, A. M. 1993. Biology, ecology, and management of the diamondback moth. Annu. Rev. Entomol. 38:275–301.
Tolrà , R. O., Poschenrieder, C., Alonso, R., Barceló, D., and Barceló, J. 2001. Influence of zinc hyperaccumulation on glucosinolates in Thlaspi caerulescens. New Phytol. 151:621–626.
Twigg, L. E. and King, D. R. 1991. The impact of fluoroacetate-bearing vegetation on native Australian fauna: A review. Oikos 61:412–430.
Wall, M. A. and Boyd, R. S. 2002. Nickel accumulation in serpentine arthropods from the Red Hills, California. Pan-Pac. Entomol. 78:168–176.
Weimin, L., Schuler, M. A., and Berenbaum, M. R. 2003. Diversification of furanocoumarin-metabolizing cytochrome P450 monooxygenases in two papilionids: Specificity and substrate encounter rate. Proc. Natl. Acad. Sci. U.S.A. 100:14593–14598.
Yildiz, D. 2004. Nicotine, its metabolism and an overview of its biological effects. Toxicon 43:619–632.
Zar, J. H. 1996. Biostatistical Analysis. Prentice-Hall, Englewood Cliffs.
Acknowledgments
The authors thank Rachel Foster for invaluable assistance with artificial diet experiments, Michael Buckman and Zandra Delamar for assistance with DBM colony maintenance, and Dr. John Odom for assistance with ICP analysis. The authors thank Dr. Debbie Folkerts and two anonymous reviewers for critically reviewing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jhee, E.M., Boyd, R.S. & Eubanks, M.D. Effectiveness of Metal–Metal and Metal–Organic Compound Combinations Against Plutella xylostella: Implications for Plant Elemental Defense. J Chem Ecol 32, 239–259 (2006). https://doi.org/10.1007/s10886-005-9000-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-9000-0