Abstract
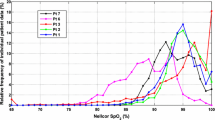
The displayed readings of Masimo pulse oximeters used in the Benefits Of Oxygen Saturation Targeting (BOOST) II and related trials in very preterm babies were influenced by trial-imposed offsets and an artefact in the calibration software. A study was undertaken to implement new algorithms that eliminate the effects of offsets and artefact. In the BOOST-New Zealand trial, oxygen saturations were averaged and stored every 10 s up to 36 weeks’ post-menstrual age. Two-hundred and fifty-seven of 340 babies enrolled in the trial had at least two weeks of stored data. Oxygen saturation distribution patterns corresponding with a +3 % or −3 % offset in the 85–95 % range were identified together with that due to the calibration artefact. Algorithms involving linear and quadratic interpolations were developed, implemented on each baby of the dataset and validated using the data of a UK preterm baby, as recorded from Masimo oximeters with the original software and a non-offset Siemens oximeter. Saturation distributions obtained were compared for both groups. There were a flat region at saturations 85–87 % and a peak at 96 % from the lower saturation target oximeters, and at 93–95 and 84 % respectively from the higher saturation target oximeters. The algorithms lowered the peaks and redistributed the accumulated frequencies to the flat regions and artefact at 87–90 %. The resulting distributions were very close to those obtained from the Siemens oximeter. The artefact and offsets of the Masimo oximeter’s software had been addressed to determine the true saturation readings through the use of novel algorithms. The implementation would enable New Zealand data be included in the meta-analysis of BOOST II trials, and be used in neonatal oxygen studies.







Similar content being viewed by others
References
Cross KW. Cost of preventing retrolental fibroplasia. Lancet. 1973;2:954–6.
Askie LM, Henderson-Smart DH. Restricted versus liberal oxygen exposure for preventing morbidity and mortality in preterm or low birth weight infants. Cochrane Database Syst Rev. 2001;4:CD001077.
Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9.
Silverman WA. A cautionary tale about supplemental oxygen: the albatross of neonatal medicine. Pediatrics. 2004;113:394–6.
Askie LM, Brocklehurst P, Darlow BA, et al. NeOProM: neonatal oxygenation prospective meta-analysis collaboration study protocol. BMC Pediatr. 2011;11:6.
Darlow BA, Marschner S, Donoghoe M, et al. Randomized controlled trial of oxygen saturation targets in very preterm infants: two year outcomes. J Pediatr. 2014;165:30–5.
Johnston ED, Boyle B, Juszczak E, et al. Oxygen targeting in preterm infants using the Masimo SET Radical pulse oximeter. Arch Dis Child Fetal Neonat Ed. 2011;96:F429–33.
Schmidt B, Whyte RK, Asztalos EV, Canadian Oxygen Trial (COT) Group, et al. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA. 2013;309:2111–20.
The BOOST II UK, Australia and New Zealand Collaborative Groups, Stenson BJ, Tarnow-Mordi WO, Darlow BA, et al. Oxygen saturation and outcomes in preterm infants. N Engl J Med. 2013;368:2094–104.
SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network, Carlo WA, Finer NN, Walsh MC, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–69.
Vaucher YE, Peralta-Carcelen M, Finer NN, et al. Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. N Engl J Med. 2012;367:2495–504.
Cunningham S, Mclntosh N, Fleck BW, et al. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995;346:1464–5.
York JR, Landers S, Kirby RS, et al. Arterial oxygen fluctuation and retinopathy of prematurity in very-low-birth-weight infants. J Perinatol. 2004;24:82–7.
Schmidt B, Whyte RK, Asztalos EV, Canadian Oxygen Trial (COT) Group, et al. Masking algorithm of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA. 2014;309:2111–20.
Acknowledgments
We are grateful to Ed Juszczak PhD, Andy King PhD and Ben Stenson MD from the BOOST II UK study group for providing us with oxygen saturation data from a UK baby, and to Nicolette McNeill and Patricia Graham for their assistance with the BOOST-NZ data. Our thanks also to Prof K. Ibrahim for his comments on paper drafts. The BOOST-NZ study was funded by a project grant from the New Zealand Health Research Council (05-145), with additional funding from the Child Health Research Foundation (Cure Kids).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. Authors confirm that they have no financial relationship with the organization that sponsored the research.
Ethics committee
Multi-region Ethics Committee of the Ministry of Health.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethical standards
The authors confirm that the experiments comply with the current laws of the country in which they were performed.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Zahari, M., Lee, D.S. & Darlow, B.A. Algorithms that eliminate the effects of calibration artefact and trial-imposed offsets of Masimo oximeter in BOOST-NZ trial. J Clin Monit Comput 30, 669–678 (2016). https://doi.org/10.1007/s10877-015-9752-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-015-9752-1