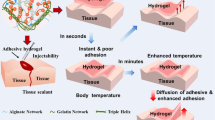
Abstract
A physical cross-linking method was used to construct an injectable hydrogel composite comprised of bioactive glass (BG) particles and polyvinyl alcohol (PVA). BG/PVA hydrogel morphology, mechanical properties, and viscoelasticity were explored. The hydrogel composite had a homogeneous and uniform distribution of BG particles, as evidenced by scanning electronic microscopy. The elastic modulus and static compressive strength were elevated by 152% and 345%, respectively, by adding 2.5 wt% BG particles to the hydrogel composite constructions. A self-standing elastic hydrogel composite with a smooth injectability was found to have a storage mould (G) more remarkable than a loss module (G″) and over the entire frequency range examined. The cell viability and live/dead assay investigation suggested the suitability of the BG/PVA hydrogel composite compared to the control PVA hydrogel groups. Adult male rats were implanted subcutaneously with the BG/PVA hydrogel composite. No inflammatory cell was found inside or over the implant after 4 weeks of implantation, indicating that the hydrogel composite was highly biocompatible. The newly constructed injectable hydrogel BG/PVA nanocomposite properties establish its ability to repair the shoulder joint head for hemiarthroplasty.









Similar content being viewed by others
References
W. Zhang, G.-J. Yang, S.-X. Wu, D.-Q. Li, Y.-B. Xu, C.-H. Ma, J.-L. Wang, and W.-W. Chen (2018). The guiding role of bone metabolism test in osteoporosis treatment. Am. J. Clin. Exp. Immunol. 7, 40.
G. Adami, K. G. Saag, A. S. Mudano, E. J. Rahn, N. C. Wright, R. C. Outman, S. L. Greenspan, A. Z. LaCroix, J. W. Nieves, and S. L. Silverman (2020). Factors associated with the contemplative stage of readiness to initiate osteoporosis treatment. Osteoporos. Int. 31, 1283–1290.
C.-H. Wu, Y.-F. Chang, C.-H. Chen, E. M. Lewiecki, C. Wüster, I. Reid, K.-S. Tsai, T. Matsumoto, L. B. Mercado-Asis, and D.-C. Chan (2019). Consensus statement on the use of bone turnover markers for short-term monitoring of osteoporosis treatment in the Asia-Pacific region. J. Clin. Densitom. 24, 3.
S. P. Schiellerup, K. Skov-Jeppesen, J. A. Windeløv, M. S. Svane, J. J. Holst, B. Hartmann, and M. M. Rosenkilde (2019). Gut hormones and their effect on bone metabolism. Potential drug therapies in future osteoporosis treatment. Front. Endocrinol. (Lausanne) 10, 75.
K. Ebina, M. Hirao, H. Tsuboi, Y. Nagayama, M. Kashii, S. Kaneshiro, A. Miyama, H. Nakaya, Y. Kunugiza, and G. Okamura (2020). Effects of prior osteoporosis treatment on early treatment response of romosozumab in patients with postmenopausal osteoporosis. Bone 140, 115574.
L. Hu, C. Yin, F. Zhao, A. Ali, J. Ma, and A. Qian (2018). Mesenchymal stem cells: cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. Int. J. Mol. Sci. 19, 360.
S. Khosla and L. C. Hofbauer (2017). Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 5, 898–907.
P. Mora-Raimundo, D. Lozano, M. Manzano, and M. Vallet-Regí (2019). Nanoparticles to knockdown osteoporosis-related gene and promote osteogenic marker expression for osteoporosis treatment. ACS Nano 13, 5451–5464.
C. Echalier, L. Valot, J. Martinez, A. Mehdi, and G. Subra (2019). Chemical cross-linking methods for cell encapsulation in hydrogels. Mater. Today Commun. 20, 100536.
S.-W. Wu, X. Liu, A. L. Miller II., Y.-S. Cheng, M.-L. Yeh, and L. Lu (2018). Strengthening injectable thermo-sensitive NIPAAm-g-chitosan hydrogels using chemical cross-linking of disulfide bonds as scaffolds for tissue engineering. Carbohydr. Polym. 192, 308–316.
S. Xia, Q. Zhang, S. Song, L. Duan, and G. Gao (2019). Bioinspired dynamic cross-linking hydrogel sensors with skin-like strain and pressure sensing behaviors. Chem. Mater. 31, 9522–9531.
Y. Zhao and Z. Sun (2017). Effects of gelatin-polyphenol and gelatin–genipin cross-linking on the structure of gelatin hydrogels. Int. J. Food Prop. 20, S2822–S2832.
N.T.-P. Nguyen, L.V.-H. Nguyen, N.M.-P. Tran, D. T. Nguyen, T.N.-T. Nguyen, H. A. Tran, N.N.-T. Dang, T. Van Vo, and T.-H. Nguyen (2019). The effect of oxidation degree and volume ratio of components on properties and applications of in situ cross-linking hydrogels based on chitosan and hyaluronic acid. Mater. Sci. Eng. C 103, 109670.
A. Ibáñez-Fonseca, T. L. Ramos, I. Gonzalez de Torre, L. I. Sánchez-Abarca, S. Muntión, F. J. Arias, M. C. Del Cañizo, M. Alonso, F. Sánchez-Guijo, and J. C. Rodríguez-Cabello (2018). Biocompatibility of two model elastin-like recombinamer-based hydrogels formed through physical or chemical cross-linking for various applications in tissue engineering and regenerative medicine. J. Tissue Eng. Regen. Med. 12, e1450–e1460.
Z. Wang, W. Hu, Y. Du, Y. Xiao, X. Wang, S. Zhang, J. Wang, and C. Mao (2020). Green gas-mediated cross-linking generates biomolecular hydrogels with enhanced strength and excellent hemostasis for wound healing. ACS Appl. Mater. Interfaces 12, 13622–13633.
W. Chen, N. Li, Y. Ma, M. L. Minus, K. Benson, X. Lu, X. Wang, X. Ling, and H. Zhu (2019). Superstrong and tough hydrogel through physical cross-linking and molecular alignment. Biomacromolecules 20, 4476–4484.
A. M. Sisso, M. O. Boit, and C. A. DeForest (2020). Self-healing injectable gelatin hydrogels for localized therapeutic cell delivery. J. Biomed. Mater. Res. Part A. 108, 1112–1121.
N. C. Negrini, N. Celikkin, P. Tarsini, S. Farè, and W. Święszkowski (2020). Three-dimensional printing of chemically cross-linked gelatin hydrogels for adipose tissue engineering. Biofabrication. 12, 25001.
K. S. Lim, B. J. Klotz, G. C. J. Lindberg, F. P. W. Melchels, G. J. Hooper, J. Malda, D. Gawlitta, and T. B. F. Woodfield (2019). Visible light cross-linking of gelatin hydrogels offers an enhanced cell microenvironment with improved light penetration depth. Macromol. Biosci. 19, 1900098.
O. Hasturk, K. E. Jordan, J. Choi, and D. L. Kaplan (2020). Enzymatically cross-linked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials. 232, 119720.
L. Zhang, J. Liu, X. Zheng, A. Zhang, X. Zhang, and K. Tang (2019). Pullulan dialdehyde cross-linked gelatin hydrogels with high strength for biomedical applications. Carbohydr. Polym. 216, 45–53.
M. Di Giuseppe, N. Law, B. Webb, R. A. Macrae, L. J. Liew, T. B. Sercombe, R. J. Dilley, and B. J. Doyle (2018). Mechanical behaviour of alginate-gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 79, 150–157.
P. Nikpour, H. Salimi-Kenari, and S. M. Rabiee (2021). Biological and bioactivity assessment of dextran nanocomposite hydrogel for bone regeneration. Prog. Biomater. 10, 271.
L. Zhou, L. Fan, F.-M. Zhang, Y. Jiang, M. Cai, C. Dai, Y.-A. Luo, L.-J. Tu, Z.-N. Zhou, and X.-J. Li (2021). Hybrid gelatin/oxidized chondroitin sulfate hydrogels incorporating bioactive glass nanoparticles with enhanced mechanical properties, mineralization, and osteogenic differentiation. Bioact. Mater. 6, 890–904.
N. Gupta, A. Singh, N. Dey, S. Chattopadhyay, J. P. Joseph, D. Gupta, M. Ganguli, and A. Pal (2021). Pathway-driven peptide-bioglass nanocomposites as the dynamic and self-healable matrix. Chem. Mater. 33, 589–599.
S. K. Kailasa, D. J. Joshi, M. R. Kateshiya, J. R. Koduru, and N. I. Malek (2022). Review on the biomedical and sensing applications of nanomaterial-incorporated hydrogels. Mater. Today Chem. 23, 100746.
A. Sadeghian, M. Kharaziha, and M. Khoroushi (2022). Osteoconductive visible light-crosslinkable nanocomposite for hard tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 632, 127761.
P. N. Christy, S. K. Basha, and V. S. Kumari (2022). Nano zinc oxide and nano bioactive glass reinforced chitosan/poly (vinyl alcohol) scaffolds for bone tissue engineering application. Mater. Today Commun. 31, 103429.
D. Vukajlovic, O. Bretcanu, and K. Novakovic (2021). Fabrication and characterization of two types of bone composites made of chitosan-genipin hydrogel and Bioglass 45S5. Open Ceram. 8, 100174.
G. Gharati, S. Shirian, S. Sharifi, E. Mirzaei, B. Bakhtirimoghadam, I. Karimi, and H. Nazari (2021). Comparison capacity of collagen hydrogel and collagen/strontium bioglass nanocomposite scaffolds with and without mesenchymal stem cells in regeneration of critical sized bone defect in a rabbit animal model. Biol. Trace Elem. Res. 200, 3176.
C. D. F. Moreira, S. M. Carvalho, R. M. Florentino, A. França, B. S. Okano, C. M. F. Rezende, H. S. Mansur, and M. M. Pereira (2019). Injectable chitosan/gelatin/bioactive glass nanocomposite hydrogels for potential bone regeneration: in vitro and in vivo analyses. Int. J. Biol. Macromol. 132, 811–821.
P. Nikpour, H. Salimi-Kenari, F. Fahimipour, S. M. Rabiee, M. Imani, E. Dashtimoghadam, and L. Tayebi (2018). Dextran hydrogels incorporated with bioactive glass-ceramic: nanocomposite scaffolds for bone tissue engineering. Carbohydr. Polym. 190, 281–294.
S. O. Ebhodaghe (2022). Hydrogel–based biopolymers for regenerative medicine applications: a critical review. Int. J. Polym. Mater. Polym. Biomater. 71, 155–172.
L. Chen, W. Wang, Z. Lin, Y. Lu, H. Chen, B. Li, Z. Li, H. Xia, L. Li, and T. Zhang (2022). Conducting molybdenum sulfide/graphene oxide/polyvinyl alcohol nanocomposite hydrogel for repairing spinal cord injury. J. Nanobiotechnol. 20, 1–17.
S. Huang, X. Hong, M. Zhao, N. Liu, H. Liu, J. Zhao, L. Shao, W. Xue, H. Zhang, and P. Zhu (2022). Nanocomposite hydrogels for biomedical applications. Bioeng Transl Med. https://doi.org/10.1002/btm2.10315.
C. D. F. Moreira, S. M. Carvalho, R. G. Sousa, H. S. Mansur, and M. M. Pereira (2018). Nanostructured chitosan/gelatin/bioactive glass in situ forming hydrogel composites as a potential injectable matrix for bone tissue engineering. Mater. Chem. Phys. 218, 304–316.
R. Dong, F. Lu, P. Liu, X. Li, R. Huang, G. Liu, and J. Chen (2022). Preparation of nanocellulose-polyvinyl alcohol composite hydrogels from Desmodium intortum (Mill.) Urb.: chemical property characterization. Ind. Crops Prod. 176, 114371.
Z. Yang, F. Zhao, W. Zhang, Z. Yang, M. Luo, L. Liu, X. Cao, D. Chen, and X. Chen (2021). Degradable photothermal bioactive glass composite hydrogel for the sequential treatment of tumor-related bone defects: from anti-tumor to repairing bone defects. Chem. Eng. J. 419, 129520.
S. Amirthalingam, S. S. Lee, M. Pandian, J. Ramu, S. Iyer, N. S. Hwang, and R. Jayakumar (2021). Combinatorial effect of nano whitlockite/nano bioglass with FGF-18 in an injectable hydrogel for craniofacial bone regeneration. Biomater. Sci. 9, 2439–2453.
J. Wu, K. Zheng, X. Huang, J. Liu, H. Liu, A. R. Boccaccini, Y. Wan, X. Guo, and Z. Shao (2019). Thermally triggered injectable chitosan/silk fibroin/bioactive glass nanoparticle hydrogels for in-situ bone formation in rat calvarial bone defects. Acta Biomater. 91, 60–71.
E. Zeimaran, S. Pourshahrestani, A. Fathi, N. A. B. Abd Razak, N. A. Kadri, A. Sheikhi, and F. Baino (2021). Advances in bioactive glass-containing injectable hydrogel biomaterials for tissue regeneration. Acta Biomater. 136, 1.
X. Bai, S. Lü, H. Liu, Z. Cao, P. Ning, Z. Wang, C. Gao, B. Ni, D. Ma, and M. Liu (2017). Polysaccharides based injectable hydrogel compositing bio-glass for cranial bone repair. Carbohydr. Polym. 175, 557–564.
P. Bargavi, R. Ramya, S. Chitra, S. Vijayakumari, R. R. Chandran, D. Durgalakshmi, P. Rajashree, and S. Balakumar (2020). Bioactive, degradable and multi-functional three-dimensional membranous scaffolds of bioglass and alginate composites for tissue regenerative applications. Biomater. Sci. 8, 4003–4025.
X. Zhang, Y. Li, Z. Ma, D. He, and H. Li (2021). Modulating degradation of sodium alginate/bioglass hydrogel for improving tissue infiltration and promoting wound healing. Bioact. Mater. 6, 3692–3704.
A. Kumar and S. S. Han (2021). Enhanced mechanical, biomineralization, and cellular response of nanocomposite hydrogels by bioactive glass and halloysite nanotubes for bone tissue regeneration. Mater. Sci. Eng. C 128, 112236.
K. Vuornos, M. Ojansivu, J. T. Koivisto, H. Häkkänen, B. Belay, T. Montonen, H. Huhtala, M. Kääriäinen, L. Hupa, and M. Kellomäki (2019). Bioactive glass ions induce efficient osteogenic differentiation of human adipose stem cells encapsulated in gellan gum and collagen type I hydrogels. Mater. Sci. Eng. C. 99, 905–918.
S. P. Sevari, F. Shahnazi, C. Chen, J. C. Mitchell, S. Ansari, and A. Moshaverinia (2020). Bioactive glass-containing hydrogel delivery system for osteogenic differentiation of human dental pulp stem cells. J. Biomed. Mater. Res. Part A. 108, 557–564.
L. Kong, Z. Wu, H. Zhao, H. Cui, J. Shen, J. Chang, H. Li, and Y. He (2018). Bioactive injectable hydrogels containing desferrioxamine and bioglass for diabetic wound healing. ACS Appl. Mater. Interfaces 10, 30103–30114.
W. Liang, X. Wu, Y. Dong, R. Shao, X. Chen, P. Zhou, and F. Xu (2021). In vivo behavior of bioactive glass-based composites in animal models for bone regeneration. Biomater Sci. https://doi.org/10.1039/D0BM01663B.
A. Gomez-Lopez, B. Grignard, I. Calvo, C. Detrembleur, and H. Sardon (2020). Monocomponent non-isocyanate polyurethane adhesives based on a sol-gel process. ACS Appl. Polym. Mater. 2, 1839–1847.
M. Yang, W. Liu, C. Jiang, S. He, Y. Xie, and Z. Wang (2018). Fabrication of superhydrophobic cotton fabric with fluorinated TiO2 sol by a green and one-step sol-gel process. Carbohydr. Polym. 197, 75–82.
A. Dehghanghadikolaei, J. Ansary, and R. Ghoreishi (2018). Sol-gel process applications: a mini-review. Proc. Nat. Res. Soc. 2, 2008.
J. Huang, L. Chen, Q. Yuan, Z. Gu, and J. Wu (2019). Tofu-Based hybrid hydrogels with antioxidant and low immunogenicity activity for enhanced wound healing. J. Biomed. Nanotechnol. 15, 1371–1383.
A. Dev, S. J. Mohanbhai, A. C. Kushwaha, A. Sood, M. N. Sardoiwala, S. R. Choudhury, and S. Karmakar (2020). κ-carrageenan-C-phycocyanin based smart injectable hydrogels for accelerated wound recovery and real-time monitoring. Acta Biomater. 109, 121–131. https://doi.org/10.1016/j.actbio.2020.03.023.
O. Domengé, H. Ragot, R. Deloux, A. Crépet, G. Revet, S. E. Boitard, A. Simon, N. Mougenot, L. David, T. Delair, A. Montembault, and O. Agbulut (2021). Efficacy of epicardial implantation of acellular chitosan hydrogels in ischemic and nonischemic heart failure: impact of the acetylation degree of chitosan. Acta Biomater. 119, 125–139. https://doi.org/10.1016/j.actbio.2020.10.045.
S. Camarero-Espinosa, B. Rothen-Rutishauser, E. J. Foster, and C. Weder (2016). Articular cartilage: from formation to tissue engineering. Biomater. Sci. 4, 734–767. https://doi.org/10.1039/C6BM00068A.
J. Sonamuthu, Y. Cai, H. Liu, M. S. M. Kasim, V. R. Vasanthakumar, B. Pandi, H. Wang, and J. Yao (2020). MMP-9 responsive dipeptide-tempted natural protein hydrogel-based wound dressings for accelerated healing action of infected diabetic wound. Int. J. Biol. Macromol. 153, 1058–1069. https://doi.org/10.1016/j.ijbiomac.2019.10.236.
M. S. Mohamed Kasim, S. Sundar, and R. Rengan (2018). Synthesis and structure of new binuclear ruthenium(II) arene benzil bis(benzoylhydrazone) complexes: Investigation on antiproliferative activity and apoptosis induction. Inorg. Chem. Front. 5, 585–596. https://doi.org/10.1039/c7qi00761b.
M. K. M. Subarkhan and R. Ramesh (2016). Ruthenium(ii) arene complexes containing benzhydrazone ligands: synthesis, structure and antiproliferative activity. Inorg. Chem. Front. 3, 1245–1255. https://doi.org/10.1039/C6QI00197A.
K. Giriraj, M. S. Mohamed Kasim, K. Balasubramaniam, S. K. Thangavel, J. Venkatesan, S. Suresh, P. Shanmugam, and C. Karri (2022). Various coordination modes of new coumarin Schiff bases toward Cobalt (III) ion: synthesis, spectral characterization, in vitro cytotoxic activity, and investigation of apoptosis. Appl. Organomet. Chem. 36, e6536. https://doi.org/10.1002/aoc.6536.
R. Pilliadugula, J. Haribabu, M. K. Mohamed Subarkhan, C. Echeverria, R. Karvembu, and N. Gopalakrishnan (2021). Effect of morphology and (Sn, Cr) doping on in vitro antiproliferation properties of hydrothermally synthesized 1D GaOOH nanostructures. J. Sci. Adv. Mater. Devices 6, 351–363. https://doi.org/10.1016/j.jsamd.2021.03.003.
G. Kalaiarasi, M. Mohamed Subarkhan, C. K. Fathima Safwana, S. Sruthi, T. Sathiya Kamatchi, B. Keerthana, and S. L. Ashok-Kumar (2022). New organoruthenium(II) complexes containing N, X-donor (X = O, S) heterocyclic chelators: synthesis, spectral characterization, in vitro cytotoxicity and apoptosis investigation. Inorg. Chim. Acta 535, 120863. https://doi.org/10.1016/j.ica.2022.120863.
S. Swaminathan, J. Haribabu, M. K. Mohamed Subarkhan, D. Gayathri, N. Balakrishnan, N. Bhuvanesh, C. Echeverria, and R. Karvembu (2021). Impact of aliphatic acyl and aromatic thioamide substituents on the anticancer activity of Ru(ii)-p-cymene complexes with acylthiourea ligands—in vitro and in vivo studies. Dalton Trans. 50, 16311–16325. https://doi.org/10.1039/D1DT02611A.
S. Balaji, M. K. Mohamed Subarkhan, R. Ramesh, H. Wang, and D. Semeril (2020). Synthesis and structure of arene Ru(II) N∧O-chelating complexes: in vitro cytotoxicity and cancer cell death mechanism. Organometallics 39, 1366–1375. https://doi.org/10.1021/acs.organomet.0c00092.
Y. Wang, J. Jin, L. Shu, T. Li, S. Lu, M. K. M. Subarkhan, C. Chen, and H. Wang (2020). New organometallic ruthenium(II) compounds synergistically show cytotoxic, antimetastatic and antiangiogenic activities for the treatment of metastatic cancer. Chem. A Eur. J. 26, 15170–15182. https://doi.org/10.1002/chem.202002970.
I. P. Grudzinski, M. Bystrzejewski, M. A. Cywinska, A. Kosmider, M. Poplawska, A. Cieszanowski, and A. Ostrowska (2013). Cytotoxicity evaluation of carbon-encapsulated iron nanoparticles in melanoma cells and dermal fibroblasts. J. Nanopart. Res. 15, 1835. https://doi.org/10.1007/s11051-013-1835-7.
W. Xu, R. Thapa, D. Liu, T. Nissinen, S. Granroth, A. Närvänen, M. Suvanto, H. A. Santos, and V.-P. Lehto (2015). Smart porous silicon nanoparticles with polymeric coatings for sequential combination therapy. Mol. Pharm. 12, 4038–4047. https://doi.org/10.1021/acs.molpharmaceut.5b00473.
Y. Shao, X. Tian, W. Hu, Y. Zhang, H. Liu, H. He, Y. Shen, F. Xie, and L. Li (2012). The properties of Gd2O3-assembled silica nanocomposite targeted nanoprobes and their application in MRI. Biomaterials 33, 6438–6446. https://doi.org/10.1016/j.biomaterials.2012.05.065.
M. Sedighi, F. Rahimi, M.-A. Shahbazi, A. H. Rezayan, H. Kettiger, T. Einfalt, J. Huwyler, and D. Witzigmann (2020). Controlled tyrosine kinase inhibitor delivery to liver cancer cells by gate-capped mesoporous silica nanoparticles. ACS Appl. Bio Mater. 3, 239–251. https://doi.org/10.1021/acsabm.9b00772.
Funding
This research received no specific grant from the public, commercial, or not-for-profit funding agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, F., Lin, C. Precise Construction of Injectable Bioactive Glass/Polyvinyl Alcohol Nanocomposite Hydrogels Promising to Repair the Shoulder Joint Head for Hemiarthroplasty. J Clust Sci 34, 1841–1851 (2023). https://doi.org/10.1007/s10876-022-02331-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02331-5