Abstract
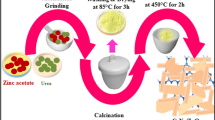
In this research work for the first time, PbO/GrO nanoporouses were prepared by direct thermal decomposition method with PbSO4 nanostructures as a precursor. The precursor was calcinated in air for 2 h at 250, 260, 270 °C. The XRD studies indicated the production of pure PbO/GrO nanocomposites after thermal decomposition. In finally the efficiency of PbO/GrO nanocomposites for photocatalyst investigation at decolorization of methylene blue using visible light irradiation has been evaluated. Samples were characterized by X-ray diffraction (XRD), atomic force microscopy, scanning electron microscopy, transmission electron microscopy and Photoluminescence spectra. The XRD results indicated that the PbO/GrO nanocomposites were synthesis without any impurities could be obtained after thermal decomposition.










Similar content being viewed by others
References
A. U. Czaja, N. Trukhan, and U. Müller (2009). Chem. Soc. Rev. 38, 1284.
H. Asadollahzadeh, M. Ranjbar, and M. A. Taher (2014). J. Indus. Eng. Chem. 20, 4321.
J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen, and J. T. Hupp (2009). Chem. Soc. Rev. 38, 1450.
U. Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt, and J. Pastré (2006). J. Mater. Chem. 16, 626.
O. Yaghi, M. O’Keeffe, N. Ockwig, H. Chae, M. Eddaoudi, and J. Kim (2003). Nature 423, 705.
X. Li, G. He, G. Xiao, H. Liu, and M. Wang (2009). J. Colloid Interface Sci. 333, 465.
F. Luo and S. R. Batten (2010). Dalton Trans. 39, 4485.
Y. M. Song, F. Luo, M. B. Luo, Z. W. Liao, G. M. Sun, X. Z. Tian, Y. Zhu, Z. J. Yuan, S. J. Liu, W. Y. Xu, and X. F. Feng (2012). Chem. Commun. 4, 1006.
B. Zhang, J. Zhong, and Z. Cheng (2011). J. Power Sources 196, 571.
H. Karami and Alipour (2009). Int. J. Electrochem. Sci. 4, 151.
C. J. Kepert, T. J. Prior, and M. J. Rosseinsky (2000). J. Am. Chem. Soc. 122, 5158.
L. Y. Xin, G. Z. Liu, X. L. Li, and L. Y. Wang (2012). Cryst. Growth Des. 12, 147.
M. Dinca and J. R. Long (2005). J. Am. Chem. Soc. 127, 9376.
Q. Sun, Y.-Q. Wang, A.-L. Cheng, K. Wang, and E.-Q. Gao (2012). Cryst. Growth Des. 12, 2234.
H.-X. Deng, C. J. Doonan, H. Furukawa, R. B. Ferreira, J. Towne, C. B. Knobler, B. Wang, and O. M. Yaghi (2010). Science 327, 846.
H.-L. Jiang, Y. Tatsu, Z.-H. Lu, and Q. Xu (2010). J. Am. Chem. Soc. 132, 5586.
K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae, and J. R. Long (2011). Chem. Rev. 112, 724.
J. R. Li, J. Sculley, and H.-C. Zhou (2011). Chem. Rev. 112, 869.
D. López, R. Buitrago, A. Sepúlveda-Escribano, F. Rodríguez- Reinoso, and F. Mondragón (2008). J. Phys. Chem. C 112, 15335.
D. Britt, D. Tranchemontagne, and O. M. Yaghi (2008). Proc. Natl. Acad. Sci. 105, 11623.
M. M. Foroughi and M. Ranjbar (2017). J. Mater. Sci. Mater. Electron. 28, 1359.
S. Ma, J. A. Fillinger, M. W. Ambrogio, J. L. Zuo, and H. C. Zhou (2007). Inorg. Chem. Commun. 10, 220.
A. Mohadesi, M. Ranjbar, and S. M. Hosseinpour-Mashkani (2014). Superlattices Microstruct. 66, 48.
X. Cao, C. Zhao, X. Lan, G. Gao, W. Qian, and Y. Guo (2007). J. Phys. Chem. C 111, 6658.
M. Ranjbar, M. A. Taher, and S. M. Hosseinpour-Mashkani (2013). J. Clust. Sci. 24, 956.
M. Ranjbar, M. A. Taher, and A. Sam (2014). J. Clust. Sci. 25, 1657.
H. Fazelirad, M. Ranjbar, M. A. Taher, and G. Sargazi (2015). J. Ind. Eng. Chem. 21, 889.
C. S. Carney, C. J. Gump, and A. W. Weimer (2006). Mater. Sci. Eng. A. 431, 1.
M. Ranjbar, M. A. Taher, and A. Sam (2015). J. Mater. Sci. Mater. Electron. 26, 8.
Acknowledgements
Authors are grateful Kerman Branch, Islamic Azad University.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Foroughi, M.M., Ranjbar, M. Graphene Oxide Doped with PbO Nanoparticles, Synthesis by Microwave Assistant Thermal Decomposition and Investigation of Optical Property. J Clust Sci 28, 2847–2856 (2017). https://doi.org/10.1007/s10876-017-1248-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-017-1248-3