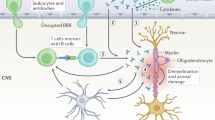
Introduction: Although myelin autoimmunity is known to be a major factor in the pathogenesis of multiple sclerosis (MS), the role of nonmyelin antigens is less clear. Given the complexity of this disease, it is possible that autoimmunity against nonmyelin antigens also has a pathogenic role. Autoantibodies against enolase and arrestin have previously been reported in MS patients. The T-cell response to these antigens, however, has not been established.
Methods: Thirty-five patients with MS were recruited, along with thirty-five healthy controls. T-cell proliferative responses against non-neuronal enolase, neuron-specific enolase (NSE), retinal arrestin, β-arrestin, and myelin basic protein were determined.
Results: MS patients had a greater prevalence of positive T-cell proliferative responses to NSE, retinal arrestin, and β-arrestin than healthy controls (p<0.0001). The proliferative response against NSE, retinal arrestin, and β-arrestin correlated with the response against myelin basic protein (p≤0.004). Furthermore, the proliferative response against retinal arrestin was correlated to β-arrestin (p<0.0001), whereas there was no such correlation between non-neuronal enolase and NSE (p = 0.23).
Discussion: There is accumulating evidence to suggest that the pathogenesis of MS involves more than just myelin autoimmunity/destruction. Autoimmunity against nonmyelin antigens may be a component of this myriad of immunopathological events. NSE, retinal arrestin, and β-arrestin are novel nonmyelin autoantigens that deserve further investigation in this respect. Autoimmunity against these antigens may be linked to neurodegeneration, defective remyelination, and predisposition to uveitis in multiple sclerosis. Further investigation of the role of these antigens in MS is warranted.





Similar content being viewed by others
REFERENCES
Ponomarenko NA, Durova OM, Vorobiev II, Belogurov AA, Telegin GB, Suchkov SV, et al.: Catalytic activity of autoantibodies toward myelin basic protein correlates with the scores on the multiple sclerosis expanded disability status scale. Immunol Lett 103(1):45–50, 2006
Raine CS: Multiple sclerosis: A pivotal role for the T cell in lesion development. Neuropathol Appl Neurobiol 17(4):265–274, 1991
Schmidt S: Candidate autoantigens in multiple sclerosis. Mult Scler 5(3):147–160, 1999
Banki K, Colombo E, Sia F, Halladay D, Mattson DH, Tatum AH, et al.: Oligodendrocyte-specific expression and autoantigenicity of transaldolase in multiple sclerosis. J Exp Med 180(5):1649–1663, 1994
Colombo E, Banki K, Tatum AH, Daucher J, Ferrante P, Murray RS, et al.: Comparative analysis of antibody and cell-mediated autoimmunity to transaldolase and myelin basic protein in patients with multiple sclerosis. J Clin Invest 99(6):1238–1250, 1997
Pratesi F, Moscato S, Sabbatini A, Chimenti D, Bombardieri S, Migliorini P: Autoantibodies specific for alpha-enolase in systemic autoimmune disorders. J Rheumatol 27(1):109–115, 2000
Gitlits VM, Toh BH, Sentry JW: Disease association, origin, and clinical relevance of autoantibodies to the glycolytic enzyme enolase. J Invest Med 49(2):138–145, 2001
Adamus G, Amundson D, Seigel GM, Machnicki M: Anti-enolase-alpha autoantibodies in cancer-associated retinopathy: Epitope mapping and cytotoxicity on retinal cells. J Autoimmun 11(6):671–677, 1998
Adamus G, Aptsiauri N, Guy J, Heckenlively J, Flannery J, Hargrave PA: The occurrence of serum autoantibodies against enolase in cancer-associated retinopathy. Clin Immunol Immunopathol 78(2):120–129, 1996
Weleber RG, Watzke RC, Shults WT, Trzupek KM, Heckenlively JR, Egan RA, et al.: Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. Am J Ophthalmol 139(5):780–794, 2005
Gorczyca WA, Ejma M, Witkowska D, Misiuk-Hojlo M, Kuropatwa M, Mulak M, et al.: Retinal antigens are recognized by antibodies present in sera of patients with multiple sclerosis. Ophthalmic Res 36(2):120–123, 2004
Forooghian F, Kertes PJ, Aptsiauri N: Probable autoimmune retinopathy in a patient with multiple sclerosis. Can J Ophthalmol 38(7):593–597, 2003
Forooghian F, Adamus G, Sproule M, Westall C, O’Connor P: Enolase autoantibodies and retinal function in multiple sclerosis patients. Graefes Arch Clin Exp Ophthalmol, 2007.
Teunissen CE, Dijkstra C, Polman C: Biological markers in CSF and blood for axonal degeneration in multiple sclerosis. Lancet Neurol 4(1):32–41, 2005
Zaffaroni M: Biological indicators of the neurodegenerative phase of multiple sclerosis. Neurol Sci 24(Suppl 5):S279–S282, 2003
Zimmer DB, Cornwall EH, Landar A, Song W: The S100 protein family: History, function, and expression. Brain Res Bull 37(4):417–429, 1995
Kondo H, Iwanaga T, Nakajima T: An immunocytochemical study on the localization of S-100 protein in the retina of rats. Cell Tissue Res 231(3):527–532, 1983
Kondo H, Takahashi H, Takahashi Y: Immunohistochemical study of S-100 protein in the postnatal development of Muller cells and astrocytes in the rat retina. Cell Tissue Res 238(3):503–508, 1984
Michetti F, Massaro A, Murazio M: The nervous system-specific S-100 antigen in cerebrospinal fluid of multiple sclerosis patients. Neurosci Lett 11(2):171–175, 1979
Petzold A, Eikelenboom MJ, Gveric D, Keir G, Chapman M, Lazeron RH, et al.: Markers for different glial cell responses in multiple sclerosis: Clinical and pathological correlations. Brain 125 (Pt 7):1462–1473, 2002
Kojima K, Berger T, Lassmann H, Hinze-Selch D, Zhang Y, Gehrmann J, et al.: Experimental autoimmune panencephalitis and uveoretinitis transferred to the Lewis rat by T lymphocytes specific for the S100 beta molecule, a calcium binding protein of astroglia. J Exp Med 180(3):817–829, 1994
Kojima K, Wekerle H, Lassmann H, Berger T, Linington C: Induction of experimental autoimmune encephalomyelitis by CD4+ T cells specific for an astrocyte protein, S100 beta. J Neural Transm Suppl 49:43–51, 1997
Broekhuyse RM, Leunissen JL, Verkley AJ: Ultrastructural localization of S-antigen in retinal structures. Curr Eye Res 4(1):73–77, 1985
McKechnie NM, Al-Mahdawi S, Dutton G, Forrester JV: Ultrastructural localization of retinal S antigen in the human retina. Exp Eye Res 42(5):479–487, 1986
Attramadal H, Arriza JL, Aoki C, Dawson TM, Codina J, Kwatra MM, et al.: Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem 267(25):17882–17890, 1992
Biousse V, Trichet C, Bloch-Michel E, Roullet E: Multiple sclerosis associated with uveitis in two large clinic-based series. Neurology 52(1):179–181, 1999
Graham EM, Francis DA, Sanders MD, Rudge P: Ocular inflammatory changes in established multiple sclerosis. J Neurol Neurosurg Psychiatry 52(12):1360–1363, 1989
Wagemans MA, Breebaart AC: Association between intermediate uveitis and multiple sclerosis. Dev Ophthalmol 23:99–105, 1992
Wacker WB, Donoso LA, Kalsow CM, Yankeelov JA, Jr, Organisciak DT: Experimental allergic uveitis. Isolation, characterization, and localization of a soluble uveitopathogenic antigen from bovine retina. J Immunol 119(6):1949–1958, 1977
Nussenblatt RB, Kuwabara T, de Monasterio FM, Wacker WB: S-antigen uveitis in primates. A new model for human disease. Arch Ophthalmol 99(6):1090–1092, 1981
de Kozak Y, Sakai J, Thillaye B, Faure JP: S antigen-induced experimental autoimmune uveo-retinitis in rats. Curr Eye Res 1(6):327–337, 1981
Sudo A, Endo M, Saitoh S: Serum anti-arrestin antibody and disease activity of multiple sclerosis—a case report of 4-year-old child. No To Hattatsu 32(5):415–419, 2000
Ohguro H, Chiba S, Igarashi Y, Matsumoto H, Akino T, Palczewski K: Beta-arrestin and arrestin are recognized by autoantibodies in sera from multiple sclerosis patients. Proc Natl Acad Sci USA 90(8):3241–3245, 1993
McDowell JH, Smith WC, Miller RL, Popp MP, Arendt A, Abdulaeva G, et al.: Sulfhydryl reactivity demonstrates different conformational states for arrestin, arrestin activated by a synthetic phosphopeptide, and constitutively active arrestin. Biochemistry 38(19):6119–6125, 1999
Winer S, Tsui H, Lau A, Song A, Li X, Cheung RK, et al.: Autoimmune islet destruction in spontaneous type 1 diabetes is not beta-cell exclusive. Nat Med 9(2):198–205, 2003
Winer S, Astsaturov I, Cheung RK, Schrade K, Gunaratnam L, Wood DD, et al.: T cells of multiple sclerosis patients target a common environmental peptide that causes encephalitis in mice. J Immunol 166(7):4751–4756, 2001
Dosch H, Cheung RK, Karges W, Pietropaolo M, Becker DJ: Persistent T cell anergy in human type 1 diabetes. J Immunol 163(12):6933–6940, 1999
McAleese SM, Dunbar B, Fothergill JE, Hinks LJ, Day IN: Complete amino acid sequence of the neurone-specific gamma isozyme of enolase (NSE) from human brain and comparison with the non-neuronal alpha form (NNE). Eur J Biochem 178(2):413–417, 1988
Kaiser E, Kuzmits R, Pregant P, Burghuber O, Worofka W: Clinical biochemistry of neuron specific enolase. Clin Chim Acta 183(1):13–31, 1989
Grigoriadis N, Ben-Hur T, Karussis D, Milonas I: Axonal damage in multiple sclerosis: A complex issue in a complex disease. Clin Neurol Neurosurg 106(3):211–217, 2004
Chitnis T, Imitola J, Khoury SJ: Therapeutic strategies to prevent neurodegeneration and promote regeneration in multiple sclerosis. Curr Drug Targets Immune Endocr Metabol Disord 5(1):11–26, 2005
Owens T: The enigma of multiple sclerosis: inflammation and neurodegeneration cause heterogeneous dysfunction and damage. Curr Opin Neurol 16(3):259–265, 2003
Deloulme JC, Helies A, Ledig M, Lucas M, Sensenbrenner M: A comparative study of the distribution of alpha- and gamma-enolase subunits in cultured rat neural cells and fibroblasts. Int J Dev Neurosci 15(2):183–194, 1997
Sensenbrenner M, Lucas M, Deloulme JC: Expression of two neuronal markers, growth-associated protein 43 and neuron-specific enolase, in rat glial cells. J Mol Med 75(9):653–663,1997
Koch M, Mostert J, Heersema D, Teelken A, De Keyser J: Plasma S100beta and NSE levels and progression in multiple sclerosis. J Neurol Sci 252(2):154–158, 2007
Adamus G, Chan CC: Experimental autoimmune uveitides: multiple antigens, diverse diseases. Int Rev Immunol 21(2–3):209–229, 2002
de Smet MD, Chan CC: Regulation of ocular inflammation–what experimental and human studies have taught us. Prog Retin Eye Res 20(6):761–797, 2001
Giordano M, D’Alfonso S, Momigliano-Richiardi P: Genetics of multiple sclerosis: linkage and association studies. Amer J Pharmacogenomics 2(1):37–58, 2002
Raja SC, Jabs DA, Dunn JP, Fekrat S, Machan CH, Marsh MJ, et al.: Pars planitis: Clinical features and class II HLA associations. Ophthalmology 106(3):594–599, 1999
Tang WM, Pulido JS, Eckels DD, Han DP, Mieler WF, Pierce K: The association of HLA-DR15 and intermediate uveitis. Amer J Ophthalmol 123(1):70–75, 1997
Hirose S, Singh VK, Donoso LA, Shinohara T, Kotake S, Tanaka T, et al.: An 18-mer peptide derived from the retinal S antigen induces uveitis and pinealitis in primates. Clin Exp Immunol 77(1):106–111, 1989
de Smet MD, Yamamoto JH, Mochizuki M, Gery I, Singh VK, Shinohara T, et al.: Cellular immune responses of patients with uveitis to retinal antigens and their fragments. Amer J Ophthalmol 110(2):135–142, 1990
Nityanand S, Singh VK, Shinohara T, Paul AK, Singh V, Agarwal PK, et al.: Cellular immune response of patients with uveitis to peptide M, a retinal S-antigen fragment. J Clin Immunol 13(5):352–358, 1993
Pette M, Fujita K, Wilkinson D, Altmann DM, Trowsdale J, Giegerich G, et al.: Myelin autoreactivity in multiple sclerosis: recognition of myelin basic protein in the context of HLA-DR2 products by T lymphocytes of multiple-sclerosis patients and healthy donors. Proc Natl Acad Sci USA 87(20):7968–7972, 1990
Wucherpfennig KW, Catz I, Hausmann S, Strominger JL, Steinman L, Warren KG: Recognition of the immunodominant myelin basic protein peptide by autoantibodies and HLA-DR2-restricted T cell clones from multiple sclerosis patients. Identity of key contact residues in the B-cell and T-cell epitopes. J Clin Invest 100(5):1114–1122, 1997
Lefkowitz RJ, Shenoy SK: Transduction of receptor signals by beta-arrestins. Science 308(5721):512–517, 2005
Mastronardi FG, daCruz LA, Wang H, Boggs J, Moscarello MA: The amount of sonic hedgehog in multiple sclerosis white matter is decreased and cleavage to the signaling peptide is deficient. Mult Scler 9(4):362–371, 2003
Seifert T, Bauer J, Weissert R, Fazekas F, Storch MK: Differential expression of sonic hedgehog immunoreactivity during lesion evolution in autoimmune encephalomyelitis. J Neuropathol Exp Neurol 64(5):404–411, 2005
John GR, Shankar SL, Shafit-Zagardo B, Massimi A, Lee SC, Raine CS, et al.: Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med 8(10):1115–1521, 2002
Jurynczyk M, Jurewicz A, Bielecki B, Raine CS, Selmaj K: Inhibition of Notch signaling enhances tissue repair in an animal model of multiple sclerosis. J Neuroimmunol 170(1–2):3–10, 2005
Mukherjee A, Veraksa A, Bauer A, Rosse C, Camonis J, Artavanis-Tsakonas S: Regulation of Notch signalling by non-visual beta-arrestin. Nat Cell Biol 7(12):1191–1201, 2005
Kalderon D: Hedgehog signaling: An Arrestin connection? Curr Biol 15(5):R175–178, 2005
Shenoy SK, Lefkowitz RJ: Receptor regulation: beta-arrestin moves up a notch. Nat Cell Biol 7(12):1159–1161, 2005
Parruti G, Peracchia F, Sallese M, Ambrosini G, Masini M, Rotilio D, et al.: Molecular analysis of human beta-arrestin-1: Cloning, tissue distribution, and regulation of expression. Identification of two isoforms generated by alternative splicing. J Biol Chem 268(13):9753–9761, 1993
Giallongo A, Oliva D, Cali L, Barba G, Barbieri G, Feo S: Structure of the human gene for alpha-enolase. Eur J Biochem 190(3):567–573, 1990
Oliva D, Cali L, Feo S, Giallongo A: Complete structure of the human gene encoding neuron-specific enolase. Genomics 10(1):157–165, 1991
McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD: Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med 11(3):335–339, 2005
Klehmet J, Shive C, Guardia-Wolff R, Petersen I, Spack EG, Boehm BO, et al.: T cell epitope spreading to myelin oligodendrocyte glycoprotein in HLA-DR4 transgenic mice during experimental autoimmune encephalomyelitis. Clin Immunol 111(1):53–60, 2004
Lehmann PV, Forsthuber T, Miller A, Sercarz EE: Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature 358(6382):155–157, 1992
McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD: Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med 182(1):75–85, 1995
Yu M, Johnson JM, Tuohy VK: A predictable sequential determinant spreading cascade invariably accompanies progression of experimental autoimmune encephalomyelitis: A basis for peptide-specific therapy after onset of clinical disease. J Exp Med 183(4):1777–1788, 1996
de Smet MD, Bitar G, Mainigi S, Nussenblatt RB: Human S-antigen determinant recognition in uveitis. Invest Ophthalmol Vis Sci 42(13):3233–3238, 2001
Tuohy VK, Yu M, Weinstock-Guttman B, Kinkel RP: Diversity and plasticity of self recognition during the development of multiple sclerosis. J Clin Invest 99(7):1682–1690, 1997
Mastronardi FG, Moscarello MA: Molecules affecting myelin stability: A novel hypothesis regarding the pathogenesis of multiple sclerosis. J Neurosci Res 80(3):301–308, 2005
Minagar A, Jy W, Jimenez JJ, Alexander JS: Multiple sclerosis as a vascular disease. Neurol Res 28(3):230–235, 2006
ACKNOWLEDGMENTS
This research was supported by a grant from the MS Scientific Foundation of Canada to FF and POC, a grant form the Canadian Institutes of Health Research to HMD, and grants from the National Eye Institute and the Karl Kirchgessner Foundation to WCS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forooghian, F., Cheung, R.K., Smith, W.C. et al. Enolase and Arrestin are Novel Nonmyelin Autoantigens in Multiple Sclerosis. J Clin Immunol 27, 388–396 (2007). https://doi.org/10.1007/s10875-007-9091-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-007-9091-1