Abstract
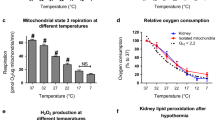
Renal artery clamping itself induces renal ischemia which subsequently causes renal cell injury and can lead to renal failure. The duration of warm ischemia that would be safe for postoperative kidney function during partial nephrectomy remains under investigations. Mitochondria play an important role in pathophysiology of ischemia-reperfusion induced kidney injury, however relation between ischemia time and mitochondrial dysfunction are not fully elucidated. Thus, the effects of renal ischemia (20 min, 40 min and 60 min) on mitochondrial functions were investigated by using in vitro rat ischemia model. Thus, electronmicroscopy showed that at short (20 min) ischemia mitochondria start to swell and the damage increases with the duration of ischemia. In accordance with this, a significant decrease in mitochondrial oxidative phosphorylation capacity was observed already after 20 min of ischemia with both, complex I dependent substrate glutamate/malate (52 %) and complex II dependent substrate succinate (44 %) which further decreased with the prolonged time of ischemia. The diminished state 3 respiration rate was associated with the decrease in mitochondrial Complex I activity and the release of cytochrome c. Mitochondrial Ca2+ uptake was diminished by 37–49 % after 20–60 min of ischemia and caspase-3 activation increased by 1.15–2.32-fold as compared to control. LDH activity changed closely with increasing time of renal ischemia. In conclusion, even short time (20 min) of warm ischemia in vitro leads to renal mitochondrial injury which increases progressively with the duration of ischemia.









Similar content being viewed by others
References
Abe K, Hayashi N, Terada H (1999) Effect of endogenous nitric oxide on energy metabolism of rat heart mitochondria during ischemia and reperfusion. Free Radic Biol Med 26:779–787
Becker F, Van PH, Hakenberg OW, Stief C, Gill I, Guazzoni G, Montorsi F, Russo P, Stockle M (2009) Assessing the impact of ischaemia time during partial nephrectomy. Eur Urol 56:625–634
Boothpur R, Brennan DC (2008) Didactic lessons from the serum lactate dehydrogenase posttransplant: a clinical vignette. Am J Transplant 8:862–865
Brezis M, Epstein FH (1993) Cellular mechanisms of acute ischemic injury in the kidney. Annu Rev Med 44:27–37
Burke TJ, Wilson DR, Levi M, Gordon JA, Arnold PE, Schrier RW (1983) Role of mitochondria in ischemic acute renal failure. Clin Exp Dial Apheresis 7:49–61
Dave KD, Saul I, Busto R, Ginsberg MD, Sick TJ, Pérez-Pinzón MA (2001) Ischemic preconditioning preserves mitochondrial function after global cerebral ischemia in rat hippocampus. J Cerebr Blood Flow & Metabolism. 21:1401–1410
Desai MM, Gill IS, Ramani AP, Spaliviero M, Rybicki L, Kaouk JH (2005) The impact of warm ischaemia on renal function after laparoscopic partial nephrectomy. BJU Int 95:377–383
Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA (2006) Calcium in cell injury and death. Annu Rev Pathol 1:405–434
Gill IS, Kamoi K, Aron M, Desai MM (2010) 800 laparoscopic partial nephrectomies: a single surgeon series. J Urol 183(1):34–41
Gonzalez-Flecha B, Boveris A (1995) Mitochondrial sites of hydrogen peroxide production in reperfused rat kidney cortex. BBA 1243:361–366
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177:751–766
Halestrap AP (2009) What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 46:821–831
Halestrap AP, Pasdois P (2009) The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta 1787:1402–1415
Hartung KJ, Jung K, Minda R, Kunz W (1985) Mitochondrial respiratory function as indicator of the ischemic injury of the rat kidney. Biomed Biochim Acta 44:1435–1443
Jassem W, Heaton ND (2004) The role of mitochondria in ischemia/reperfusion injury in organ transplantation. Kidney Int 66:514–517
Kroemer G, Reed JC (2000) Mitochondrial control of cell death. Nat Med 6:513–519
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (1997) Cytochrome c and dATP-dependent formation of apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–489
Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, De ZF, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S (2013) Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A 110:12024–12029
Liu X, Kim CN, YangJ JR, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147–157
Lukyanova LD (2013) Mitochondrial signaling in hypoxia. Open Journal of Endocrine and Metabolic Diseases 3:20–32
Marshall KD, Edwards MA, Krenz M, Davis JW, Baines CP (2014) Proteomic mapping of proteins released during necrosis and apoptosis from cultured neonatal cardiac myocytes. Am J Physiol Cell Physiol 306:C639–C647
Parekh DJ, Weinberg JM, Ercole B, Torkko KC, Hilton W, Bennett M, Devarajan P, Venkatachalam MA (2013) Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol 24:506–517
Piper HM, Sezer O, Schleyer M, Schwartz P, Hütter JF, Spieckermann PG (1985) Development of ischemia-induced damage in defined mitochondrial subpopulations. J Mol Cell Cardio. 17:885–896
Ridgway RL (1986) Flat, adherent well-contrasted semithin plastic sections for light microscopy. Stain Technol 61:253–255
Rouslin W (1983) Mitochondrial complexes I. II, III, IV, and V in Myocardial ischemia and Autolysis Am J Physiol Heart and Circ Physiol 244:H743–H748
Thompson RH, Frank I, Lohse CM, Saad IR, Fergany A, Zincke H, Leibovich BC, Blute ML, Novick AC (2007) The impact of ischemia time during open nephron sparing surgery on solitary kidneys: a multi-institutional study. J Urol 177:471–476
Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, Gill IS, Blute ML, Campbell SC (2010) Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol 58:340–345
Tirapelli LF, Bagnato VS, Tirapelli DP, Kurachi C, Barione DF, Tucci S, Suaid HJ, Cologna AJ, Martins AC (2008) Renal ischemia in rats: mitochondria function and laser autofluorescence. Transplant Proc 40:1679–1684
Volpe A, Blute ML, Ficarra V, Gill IS, Kutikov A, Porpiglia F, Rogers C, Touijer KA, Van PH, Thompson RH (2015) Renal ischemia and function after partial nephrectomy: a collaborative review of the literature. Eur Urol. doi:10.1016/j.eururo.2015.01.025
Webster KA (2012) Mitochondrial membrane permeabilization and cell death during myocardial infarction: roles of calcium and reactive oxygen species. Futur Cardiol 8:863–884
Yang B, Jain S, Pawluczyk IZ, Imtiaz S, Bowley L, Ashra SY, Nicholson ML (2005) Inflammation and caspase activation in long-term renal ischemia/reperfusion injury and immunosuppression in rats. Kidney Int 68:2050–2067
Zager RA, Johnson AC, Becker K (2013) Renal cortical lactate dehydrogenase: a useful, accurate, quantitative marker of tubular injury and acute renal failure. PLoS One 8:e66776
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
All authors read and approved final manuscript.
Rights and permissions
About this article
Cite this article
Baniene, R., Trumbeckas, D., Kincius, M. et al. Short ischemia induces rat kidney mitochondria dysfunction. J Bioenerg Biomembr 48, 77–85 (2016). https://doi.org/10.1007/s10863-016-9643-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-016-9643-2