Abstract
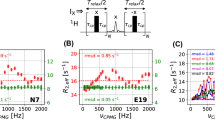
A methyl Transverse Relaxation Optimized Spectroscopy (methyl-TROSY) based, multiple quantum (MQ) 13C Carr-Purcell-Meiboom-Gill (CPMG) relaxation dispersion NMR experiment is described. The experiment is derived from the previously developed MQ 13C–1H CPMG scheme (Korzhnev in J Am Chem Soc 126: 3964–73, 2004) supplemented with a CPMG train of refocusing 1H pulses applied with constant frequency and synchronized with the 13C CPMG pulse train. The optimal 1H ‘decoupling’ scheme that minimizes the amount of fast-relaxing methyl MQ magnetization present during CPMG intervals, makes use of an XY-4 phase cycling of the refocusing composite 1H pulses. For small-to-medium sized proteins, the MQ 13C CPMG experiment has the advantage over its single quantum (SQ) 13C counterpart of significantly reducing intrinsic, exchange-free relaxation rates of methyl coherences. For high molecular weight proteins, the MQ 13C CPMG experiment eliminates complications in the interpretation of MQ 13C–1H CPMG relaxation dispersion profiles arising from contributions to exchange from differences in methyl 1H chemical shifts between ground and excited states. The MQ 13C CPMG experiment is tested on two protein systems: (1) a triple mutant of the Fyn SH3 domain that interconverts slowly on the chemical shift time scale between the major folded state and an excited state folding intermediate; and (2) the 82-kDa enzyme Malate Synthase G (MSG), where chemical exchange at individual Ile δ1 methyl positions occurs on a much faster time-scale.




Similar content being viewed by others
References
Bax A, Griffey RH, Hawkings BL (1983) Correlation of proton and nitrogen-15 chemical shifts by multiple quantum NMR. J Magn Reson 55:301–315
Carr HY, Purcell EM (1954) Effects of diffusion on free precession in nuclear magnetic resonance experiments. Phys Rev 4:630–638
Gullion T, Baker DB, Conradi MS (1990) New, compensated Carr-Purcell sequences. J Magn Reson 89:479–484
Korzhnev DM, Kloiber K, Kanelis V, Tugarinov V, Kay LE (2004a) Probing slow dynamics in high molecular weight proteins by methyl-TROSY NMR spectroscopy: application to a 723-residue enzyme. J Am Chem Soc 126:3964–3973
Korzhnev DM, Kloiber K, Kay LE (2004b) Multiple-quantum relaxation dispersion NMR spectroscopy probing millisecond time-scale dynamics in proteins: theory and application. J Am Chem Soc 126:7320–7329
Korzhnev DM, Mittermaier AK, Kay LE (2005) Cross-correlated spin relaxation effects in methyl 1H CPMG-based relaxation dispersion experiments: complications and a simple solution. J Biomol NMR 31:337–342
Levitt MH, Freeman R (1981) Compensation for pulse imperfections in NMR spin-echo experiments. J Magn Reson 43:65–80
Libich DS, Tugarinov V, Clore GM (2015) Intrinsic unfoldase/foldase activity of the chaperonin groel directly demonstrated using multinuclear relaxation-based NMR. Proc Natl Acad Sci USA 112:8817–8823
Lundström P, Vallurupalli P, Religa TL, Dahlquist FW, Kay LE (2007) A single-quantum methyl 13C-relaxation dispersion experiment with improved sensitivity. J Biomol NMR 38:79–88
Marion D, Ikura M, Tschudin R, Bax A (1989) Rapid recording of 2D NMR spectra without phase cycling. Application to the study of hydrogen exchange in proteins. J Magn Reson 85:393–399
Meiboom S, Gill D (1958) Modified spin-echo method for measuring nuclear relaxation times. Rev Sci Instrum 29:688–691
Mueller L (1979) Sensitivity enhanced detection of weak nuclei using heteronuclear multiple quantum coherence. J Am Chem Soc 101:4481–4484
Mulder FAA, Hon B, Muhandiram DR, Dahlquist FW, Kay LE (2000) Flexibility and ligand exchange in a buried cavity mutant of T4 lysozyme studied by multinuclear NMR. Biochemistry 39:12614–12622
Neudecker P, Robustelli P, Cavalli A, Walsh P, Lundstrom P, Zarrine-Afsar A, Sharpe S, Vendruscolo M, Kay LE (2012) Structure of an intermediate state in protein folding and aggregation. Science 336:362–366
Ollerenshaw JE, Tugarinov V, Kay LE (2003) Methyl TROSY: explanation and experimental verification. Magn Reson Chem 41:843–852
Parker MJ, Clarke AR (1997) Amide backbone and water-related H/D isotope effects on the dynamics of a protein folding reaction. Biochemistry 36:5786–5794
Pica A, Graziano G (2018) Effect of heavy water on the conformational stability of globular proteins. Biopolymers 109:e23076
Religa TL, Sprangers R, Kay LE (2010) Dynamic regulation of archaeal proteasome gate opening as studied by TROSY NMR. Science 328:98–102
Rosenzweig R, Kay LE (2014) Bringing dynamic molecular machines into focus by methyl-TROSY NMR. Annu Rev Biochem 83:291–315
Ruschak AM, Kay LE (2010) Methyl groups as probes of supra-molecular structure, dynamics and function. J Biomol NMR 46:75–87
Schütz S, Sprangers R (2020) Methyl TROSY spectroscopy: a versatile NMR approach to study challenging biological systems. Prog Nucl Magn Reson Spectrosc 116:56–84
Shaka AJ, Keeler J, Frenkiel T, Freeman R (1983) An improved sequence for broadband decoupling: Waltz-16. J Magn Reson 52:335–338
Stadmiller SS, Pielak GJ (2018) Enthalpic stabilization of an SH3 domain by D2O. Protein Sci 27:1710–1716
Tugarinov V, Hwang PM, Ollerenshaw JE, Kay LE (2003) Cross-correlated relaxation enhanced 1H–13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J Am Chem Soc 125:10420–10428
Tugarinov V, Hwang PM, Kay LE (2004) Nuclear magnetic resonance spectroscopy of high-molecular-weight proteins. Annu Rev Biochem 73:107–146
Tugarinov V, Kay LE (2007) Separating degenerate 1H transitions in methyl group probes for single-quantum 1h-cpmg relaxation dispersion NMR spectroscopy. J Am Chem Soc 129:9514–9521
Tugarinov V, Okuno Y, Torricella F, Karamanos TK, Clore GM (2022) A “steady-state” relaxation dispersion nuclear magnetic resonance experiment for studies of chemical exchange in degenerate 1H transitions of methyl groups. J Phys Chem Lett 13:11271–11279
Yuwen T, Huang R, Vallurupalli P, Kay LE (2019) A methyl-TROSY-based 1H relaxation dispersion experiment for studies of conformational exchange in high molecular weight proteins. Angew Chem Int Ed Engl 58:6250–6254
Acknowledgements
We thank Prof. D.F. Hansen (UCL, London, UK) for useful suggestions, Drs. F. Torricella (NIDDK, NIH) and A. Ceccon (University of Bolzano, Italy) for preparation of the NMR samples of Fyn SH3VPL and MSG, respectively, and Drs. Jinfa Ying and Dan Garrett (NIDDK, NIH) for technical support. This work was supported by the Intramural Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (DK029023 to G.M.C.).
Author information
Authors and Affiliations
Contributions
V.T. and G.M.C. designed research; V.T. and J.B. performed research; V.T. analyzed the data; V.T. and G.M.C. wrote the paper. All authors reviewed the manuscript
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10858_2023_413_MOESM1_ESM.pdf
Supplementary file1 (PDF 1222 KB)— Figures S1-S3 comparing methyl relaxation dispersion profiles obtained with different experimental schemes for Fyn SH3VPL and MSG. Table S1 listing exchange parameters of individual Ile δ1 methyl sites of MSG. ‘Materials and Methods’ describing NMR sample conditions and acquisition parameters of NMR experiments. Bruker pulse code for the experiment in Figure 1 and the associated 1H ‘decoupling’ (composite pulse decoupling) schemes. A Matlab® script for calculation of artifact locations in XY-4-cycled 1H ‘decoupling’ schemes (Figure 2, Right panels)
Rights and permissions
About this article
Cite this article
Tugarinov, V., Baber, J.L. & Clore, G.M. A methyl-TROSY based 13C relaxation dispersion NMR experiment for studies of chemical exchange in proteins. J Biomol NMR 77, 83–91 (2023). https://doi.org/10.1007/s10858-023-00413-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-023-00413-8