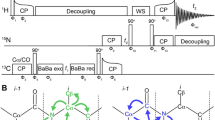
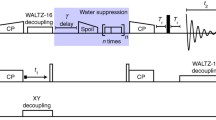
Abstract
We present a new solid-state NMR proton-detected three-dimensional experiment dedicated to the observation of protein proton side chain resonances in nano-liter volumes. The experiment takes advantage of very fast magic angle spinning and double quantum 13C–13C transfer to establish efficient (H)CCH correlations detected on side chain protons. Our approach is demonstrated on the HET-s prion domain in its functional amyloid fibrillar form, fully protonated, with a sample amount of less than 500 µg using a MAS frequency of 70 kHz. The majority of aliphatic and aromatic side chain protons (70%) are observable, in addition to Hα resonances, in a single experiment providing a complementary approach to the established proton-detected amide-based multidimensional solid-state NMR experiments for the study and resonance assignment of biosolid samples, in particular for aromatic side chain resonances.






Similar content being viewed by others
References
Agarwal V, Reif B (2008) Residual methyl protonation in perdeuterated proteins for multi-dimensional correlation experiments in MAS solid-state NMR spectroscopy. J Magn Reson 194:16–24
Andreas LB, Reese M, Eddy MT, Gelev V, Ni QZ, Miller EA, Emsley L, Pintacuda G, Chou JJ, Griffin RG (2015a) Structure and mechanism of the influenza A M218-60 dimer of dimers. J Am Chem Soc 137:14877–14886
Andreas LB, Le Marchand T, Jaudzems K, Pintacuda G (2015b) High-resolution proton-detected NMR of proteins at very fast MAS. J Magn Reson 253:36–49
Andreas LB, Stanek J, Le Marchand T, Bertarello A, Cala-De Paepe D, Lalli D, Krejcikova M, Doyen C, Oster C, Knott B, Wegner S, Engelke F, Felli IC, Pierattelli R, Dixon NE, Emsley L, Herrmann T, Pintacuda G (2015c) Protein residue linking in a single spectrum for magic-angle spinning NMR assignment. J Biomol NMR 62:253–261
Asami S, Reif B (2013) Proton-detected solid-state NMR spectroscopy at aliphatic sites: application to crystalline systems. Acc Chem Res 46:2089–2097
Asami S, Szekely K, Schanda P, Meier BH, Reif B (2012) Optimal degree of protonation for (1)H detection of aliphatic sites in randomly deuterated proteins as a function of the MAS frequency. J Biomol NMR 54:155–168
Baker LA, Baldus M (2014) Characterization of membrane protein function by solid-state NMR spectroscopy. Curr Opin Struct Biol 27:48–55
Balguerie A, Reis DS, Ritter C, Chaignepain S, Coulary-Salin B, Forge V, Bathany K, Lascu I, Schmitter J-M, Riek R, Saupe SJ (2003) Domain organization and structure-function relationship of the HET-s prion protein of Podospora anserina. EMBO J 22:2071–2081
Barbet-Massin E, Pell AJ, Retel JS, Andreas LB, Jaudzems K, Franks WT, Nieuwkoop AJ, Hiller M, Higman V, Guerry P, Bertarello A, Knight MJ, Felletti M, Le Marchand T, Kotelovica S, Akopjana I, Tars K, Stoppini M, Bellotti V, Bolognesi M, Ricagno S, Chou JJ, Griffin RG, Oschkinat H, Lesage A, Emsley L, Herrmann T, Pintacuda G (2014) Rapid proton-detected NMR assignment for proteins with fast magic angle spinning. J Am Chem Soc 136:12489–12497
Bloembergen N (1949) On the interaction of nuclear spins in a crystalline lattice. Physica 15:386–426
Brown LS, Ladizhansky V (2015) Membrane proteins in their native habitat as seen by solid-state NMR spectroscopy. Protein Sci 24:1333–1346
Castellani F, van Rossum B, Diehl A, Schubert M, Rehbein K, Oschkinat H (2002) Structure of a protein determined by solid-state magic-angle-spinning NMR spectroscopy. Nature 420:98–102
Chevelkov V, van Rossum BJ, Castellani F, Rehbein K, Diehl A, Hohwy M, Steuernagel S, Engelke F, Oschkinat H, Reif B (2003) 1H detection in MAS solid-state NMR spectroscopy of biomacromolecules employing pulsed field gradients for residual solvent suppression. J Am Chem Soc 125:7788–7789
Chevelkov V, Rehbein K, Diehl A, Reif B (2006) Ultrahigh resolution in proton solid-state NMR spectroscopy at high levels of deuteration. Angew Chem Int Ed Engl 45:3878–3881
Colvin MT, Silvers R, Ni QZ, Can TV, Sergeyev I, Rosay M, Donovan KJ, Michael B, Wall J, Linse S, Griffin RG (2016) Atomic resolution structure of monomorphic abeta42 amyloid fibrils. J Am Chem Soc 138:9663–9674
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Detken A, Hardy EH, Ernst M, Kainosho M, Kawakami T, Aimoto S, Meier BH (2001) Methods for sequential resonance assignment in solid, uniformly 13C, 15N labelled peptides: quantification and application to antamanide. J Biomol NMR 20:203–221
Fricke P, Chevelkov V, Zinke M, Giller K, Becker S, Lange A (2017) Backbone assignment of perdeuterated proteins by solid-state NMR using proton detection and ultrafast magic-angle spinning. Nat Protoc 12:764–782
Goldbourt A (2013) Biomolecular magic-angle spinning solid-state NMR: recent methods and applications. Curr Opin Biotechnol 24:705–715
Hong M (1999a) Determination of multiple φ-torsion angles in proteins by selective and extensive (13)C labeling and two-dimensional solid-state NMR. J Magn Reson 139:389–401
Hong M (1999b) Resonance assignment of 13C/15N labeled solid proteins by two- and three-dimensional magic-angle-spinning NMR. J Biomol NMR 15:1–14
Hoop CL, Lin HK, Kar K, Magyarfalvi G, Lamley JM, Boatz JC, Mandal A, Lewandowski JR, Wetzel R, van der Wel PC (2016) Huntingtin exon 1 fibrils feature an interdigitated beta-hairpin-based polyglutamine core. Proc Natl Acad Sci USA 113:1546–1551
Huber M, Hiller S, Schanda P, Ernst M, Bockmann A, Verel R, Meier BH (2011) A proton-detected 4D solid-state NMR experiment for protein structure determination. Chemphyschem 12:915–918
Kaur H, Lakatos-Karoly A, Vogel R, Noll A, Tampe R, Glaubitz C (2016) Coupled ATPase-adenylate kinase activity in ABC transporters. Nat Commun 7:13864
Knight MJ, Webber AL, Pell AJ, Guerry P, Barbet-Massin E, Bertini I, Felli IC, Gonnelli L, Pierattelli R, Emsley L, Lesage A, Herrmann T, Pintacuda G (2011) Fast resonance assignment and fold determination of human superoxide dismutase by high-resolution proton-detected solid-state MAS NMR spectroscopy. Angew Chem Int Ed Engl 50:11697–11701
Kulminskaya N, Vasa SK, Giller K, Becker S, Kwan A, Sunde M, Linser R (2016) Access to side-chain carbon information in deuterated solids under fast MAS through non-rotor-synchronized mixing. Chem Commun 52:268–271
Linser R (2017) Solid-state NMR spectroscopic trends for supramolecular assemblies and protein aggregates. Solid State Nucl Magn Reson 87:45–53
Linser R, Bardiaux B, Higman V, Fink U, Reif B (2011) Structure calculation from unambiguous long-range amide and methyl 1H-1H distance restraints for a microcrystalline protein with MAS solid-state NMR spectroscopy. J Am Chem Soc 133:5905–5912
Loquet A, Habenstein B, Lange A (2013) Structural investigations of molecular machines by solid-state NMR. Acc Chem Res 46:2070–2079
Luca S, Filippov DV, van Boom JH, Oschkinat H, de Groot HJ, Baldus M (2001) Secondary chemical shifts in immobilized peptides and proteins: a qualitative basis for structure refinement under magic angle spinning. J Biomol NMR 20:325–331
McDermott A (2009) Structure and dynamics of membrane proteins by magic angle spinning solid-state NMR. Annu Rev Biophys 38:385–403
McDermott AE, Creuzet FJ, Kolbert AC, Griffin RG (1992) High-resolution magic-angle-spinning NMR spectra of protons in deuterated solids. J Magn Reson 98:408–413
McDermott A, Polenova T, Bockmann A, Zilm KW, Paulson EK, Martin RW, Montelione GT (2000) Partial NMR assignments for uniformly (13C, 15N)-enriched BPTI in the solid state. J Biomol NMR 16:209–219
Meier BH, Bockmann A (2015) The structure of fibrils from ‘misfolded’ proteins. Curr Opin Struct Biol 30:43–49
Meier BH, Riek R, Bockmann A (2017) Emerging structural understanding of amyloid fibrils by solid-state NMR. Trends Biochem Sci 42:777–787
Moreira IS, Martins JM, Ramos RM, Fernandes PA, Ramos MJ (2013) Understanding the importance of the aromatic amino-acid residues as hot-spots. Biochim Biophys Acta 1834:404–414
Nishiyama Y, Zhang R, Ramamoorthy A (2014) Finite-pulse radio frequency driven recoupling with phase cycling for 2D (1)H/(1)H correlation at ultrafast MAS frequencies. J Magn Reson 243:25–32
Pauli J, Baldus M, van Rossum B, de Groot H, Oschkinat H (2001) Backbone and side-chain 13C and 15N signal assignments of the alpha-spectrin SH3 domain by magic angle spinning solid-state NMR at 17.6 T. ChemBioChem 2:272–281
Paulson EK, Morcombe CR, Gaponenko V, Dancheck B, Byrd RA, Zilm KW (2003) Sensitive high resolution inverse detection NMR spectroscopy of proteins in the solid state. J Am Chem Soc 125:15831–15836
Penzel S, Smith AA, Agarwal V, Hunkeler A, Org ML, Samoson A, Bockmann A, Ernst M, Meier BH (2015) Protein resonance assignment at MAS frequencies approaching 100 kHz: a quantitative comparison of J-coupling and dipolar-coupling-based transfer methods. J Biomol NMR 63:165–186
Qiang W, Yau WM, Lu JX, Collinge J, Tycko R (2017) Structural variation in amyloid-beta fibrils from Alzheimer’s disease clinical subtypes. Nature 541:217–221
Reif B, Griffin RG (2003) 1H detected 1H,15N correlation spectroscopy in rotating solids. J Magn Reson 160:78–83
Reif B, Jaroniec CP, Rienstra CM, Hohwy M, Griffin RG (2001) 1H-1H MAS correlation spectroscopy and distance measurements in a deuterated peptide. J Magn Reson 151:320–327
Saalwachter K, Lange F, Matyjaszewski K, Huang CF, Graf R (2011) BaBa-xy16: robust and broadband homonuclear DQ recoupling for applications in rigid and soft solids up to the highest MAS frequencies. J Magn Reson 212:204–215
Siemer AB, Arnold AA, Ritter C, Westfeld T, Ernst M, Riek R, Meier BH (2006a) Observation of highly flexible residues in amyloid fibrils of the HET-s prion. J Am Chem Soc 128:13224–13228
Siemer AB, Ritter C, Steinmetz MO, Ernst M, Riek R, Meier BH (2006b) 13C, 15N resonance assignment of parts of the HET-s prion protein in its amyloid form. J Biomol NMR 34:75–87
Sinnige T, Daniels M, Baldus M, Weingarth M (2014) Proton clouds to measure long-range contacts between nonexchangeable side chain protons in solid-state NMR. J Am Chem Soc 136:4452–4455
Stanek J, Andreas LB, Jaudzems K, Cala D, Lalli D, Bertarello A, Schubeis T, Akopjana I, Kotelovica S, Tars K, Pica A, Leone S, Picone D, Xu ZQ, Dixon NE, Martinez D, Berbon M, El Mammeri N, Noubhani A, Saupe S, Habenstein B, Loquet A, Pintacuda G (2016) NMR spectroscopic assignment of backbone and side-chain protons in fully protonated proteins: microcrystals, sedimented assemblies, and amyloid fibrils. Angew Chem Int Ed Engl 55:15504–15509
Struppe J, Quinn CM, Lu M, Wang M, Hou G, Lu X, Kraus J, Andreas LB, Stanek J, Lalli D, Lesage A, Pintacuda G, Maas W, Gronenborn AM, Polenova T (2017) Expanding the horizons for structural analysis of fully protonated protein assemblies by NMR spectroscopy at MAS frequencies above 100 kHz. Solid State Nucl Magn Reson 87:117–125
Sun BQ, Rienstra CM, Costa PR, Williamson JR, Griffin RG (1997) 3D 15N – 13C – 13C chemical shift correlation spectroscopy in rotating solids. J Am Chem Soc 119:8540–8546
Takegoshi K, Nakamura S, Terao T (2001) 13C–1H dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem Phys Lett 344:631–637
Tang M, Comellas G, Rienstra CM (2013) Advanced solid-state NMR approaches for structure determination of membrane proteins and amyloid fibrils. Acc Chem Res 46:2080–2088
Tuttle MD, Comellas G, Nieuwkoop AJ, Covell DJ, Berthold DA, Kloepper KD, Courtney JM, Kim JK, Barclay AM, Kendall A, Wan W, Stubbs G, Schwieters CD, Lee VM, George JM, Rienstra CM (2016) Solid-state NMR structure of a pathogenic fibril of full-length human alpha-synuclein. Nat Struct Mol Biol 23:409–415
Tycko R (2016) Molecular structure of aggregated amyloid-beta: insights from solid-state nuclear magnetic resonance. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a024083
Van Melckebeke H, Wasmer C, Lange A, Ab E, Loquet A, Bockmann A, Meier BH (2010) Atomic-resolution three-dimensional structure of HET-s(218–289) amyloid fibrils by solid-state NMR spectroscopy. J Am Chem Soc 132:13765–13775
van der Wel PCA (2017) Insights into protein misfolding and aggregation enabled by solid-state NMR spectroscopy. Solid State Nucl Magn Reson 88:1–14
Vasa SK, Rovo P, Giller K, Becker S, Linser R (2016) Access to aliphatic protons as reporters in non-deuterated proteins by solid-state NMR. Phys Chem Chem Phys 18:8359–8363
Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas M, Ulrich EL, Markley JL, Ionides J, Laue ED (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59:687–696
Walti MA, Ravotti F, Arai H, Glabe CG, Wall JS, Bockmann A, Guntert P, Meier BH, Riek R (2016) Atomic-resolution structure of a disease-relevant Abeta(1–42) amyloid fibril. Proc Natl Acad Sci USA 113:E4976–E49784
Wasmer C, Lange A, Van Melckebeke H, Siemer AB, Riek R, Meier BH (2008) Amyloid fibrils of the HET-s(218–289) prion form a beta solenoid with a triangular hydrophobic core. Science 319:1523–1526
Xiang S, Biernat J, Mandelkow E, Becker S, Linser R (2016) Backbone assignment for minimal protein amounts of low structural homogeneity in the absence of deuteration. Chem Commun 52:4002–4005
Xiao Y, Ma B, McElheny D, Parthasarathy S, Long F, Hoshi M, Nussinov R, Ishii Y (2015) Abeta(1–42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat Struct Mol Biol 22:499–505
Xue K, Sarkar R, Motz C, Asami S, Camargo DCR, Decker V, Wegner S, Tosner Z, Reif B (2017) Limits of resolution and sensitivity of proton detected MAS solid-state NMR experiments at 111 kHz in deuterated and protonated proteins. Sci Rep 7:7444
Yan S, Suiter CL, Hou G, Zhang H, Polenova T (2013) Probing structure and dynamics of protein assemblies by magic angle spinning NMR spectroscopy. Acc Chem Res 46:2047–2058
Zhang R, Duong NT, Nishiyama Y, Ramamoorthy A (2017) 3D double-quantum/double-quantum exchange spectroscopy of protons under 100 kHz magic angle spinning. J Phys Chem B 121:5944
Zhou DH, Shea JJ, Nieuwkoop AJ, Franks WT, Wylie BJ, Mullen C, Sandoz D, Rienstra CM (2007) Solid-state protein-structure determination with proton-detected triple-resonance 3D magic-angle-spinning NMR spectroscopy. Angew Chem Int Ed Engl 46:8380–8383
Acknowledgements
We acknowledge financial support from the European Research Council (ERC) under the European Unions Horizon 2020 research and innovation programme (ERC-2015-StG GA no. 639020 to A.L.), IdEx Bordeaux (Chaire d’Installation to B.H., ANR-10-IDEX-03-02), the ANR (ANR-14-CE09-0020-01 to A.L., ANR-13-PDOC-0017-01 to B.H. and ANR-17-CE11-0035 to S.J.S) and the CNRS (IR-RMN FR3050). This work has benefited from the facilities and expertise of the Biophysical and Structural Chemistry Platform (BPCS, UMS3033).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tolchard, J., Pandey, M.K., Berbon, M. et al. Detection of side-chain proton resonances of fully protonated biosolids in nano-litre volumes by magic angle spinning solid-state NMR. J Biomol NMR 70, 177–185 (2018). https://doi.org/10.1007/s10858-018-0168-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-018-0168-3