Abstract
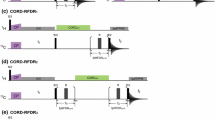
Described here is a set of three-dimensional (3D) NMR experiments that rely on CACA-TOCSY magnetization transfer via the weak \( ^{ 3} {\text{J}}_{{{\text{C}}\alpha {\text{C}}\alpha }} \) coupling. These pulse sequences, which resemble recently described 13C detected CACA-TOCSY (Takeuchi et al. 2010) experiments, are recorded in 1H2O, and use 1H excitation and detection. These experiments require alternate 13C-12C labeling together with perdeuteration, which allows utilizing the small \( ^{ 3} {\text{J}}_{{{\text{C}}\alpha {\text{C}}\alpha }} \) scalar coupling that is otherwise masked by the stronger 1JCC couplings in uniformly 13C labeled samples. These new experiments provide a unique assignment ladder-mark that yields bidirectional supra-sequential information and can readily straddle proline residues. Unlike the conventional HNCA experiment, which contains only sequential information to the \( ^{ 1 3} {\text{C}}^{\alpha } \) of the preceding residue, the 3D hnCA-TOCSY-caNH experiment can yield sequential correlations to alpha carbons in positions i−1, i + 1 and i−2. Furthermore, the 3D hNca-TOCSY-caNH and Hnca-TOCSY-caNH experiments, which share the same magnetization pathway but use a different chemical shift encoding, directly couple the 15N-1H spin pair of residue i to adjacent amide protons and nitrogens at positions i−2, i−1, i + 1 and i + 2, respectively. These new experimental features make protein backbone assignments more robust by reducing the degeneracy problem associated with the conventional 3D NMR experiments.




Similar content being viewed by others
References
Bermel W, Bertini I, Felli IC, Piccioli M, Pierattelli R (2006) 13C-detected protonless NMR spectroscopy of proteins in solution. Prog Nucl Magn Res Spec 48:25–45
Emsley L, Bodenhausen G (1992) Optimization of shaped selective pulses for NMR using a quaternion description of their overall propagators. J Magn Reson 97:135–148
Enkhmandakh B, Makeyev AV, Bayarsaihan D (2006) The role of the proline-rich domain of Ssdp1 in the modular architecture of the vertebrate head organizer. Proc Natl Acad Sci USA 103:11631–11636
Ferentz AE, Wagner G (2000) NMR spectroscopy: a multifaceted approach to macromolecular structure. Q Rev Biophys 33:29–65
Frueh DP, Arthanari H, Wagner G (2005) Unambiguous assignment of NMR protein backbone signals with a time-shared triple-resonance experiment. J Biomol NMR 33:187–196
Frueh DP, Sun ZY, Vosburg DA, Walsh CT, Hoch JC, Wagner G (2006) Non-uniformly sampled double-TROSY hNcaNH experiments for NMR sequential assignments of large proteins. J Am Chem Soc 128:5757–5763
Goddard TD, Kneller DG (2006) SPARKY 3—NMR Assignment and Integration Software. University of California, San Francisco
Grzesiek S, Bax A (1993) The importance of not saturating water in protein NMR. Application to sensitivity enhancement and NOE measurements. J Am Chem Soc 115:12593–12594
Grzesiek S, Anglister J, Bax A (1993) Correlation of backbone amide and aliphatic side-chain resonances in 13C/15N-enriched proteins by isotropic mixing of 13C Magnetization. J Magn Reson B 101:114–119
Hiller S, Arthanari H, Wagner G (2009) The T-lock: automated compensation of radio-frequency induced sample heating. J Biomol NMR 44:69–76
Kadkhodaie M, Rivas O, Tan M, Mohebbi A, Shaka AJ (1991) Broadband homonuclear cross polarization using flip-flop spectroscopy. J Magn Reson 91:437–443
Lee D, Hilty C, Wider G, Wüthrich K (2006) Effective rotational correlation times of proteins from NMR relaxation interference. J Magn Reson 178:72–76
LeMaster DM, Kushlan DM (1996) Dynamical mapping of E. coli thioredoxin via 13C NMR relaxation analysis. J Am Chem Soc 118:9255–9264
Lin Y, Wagner G (1999) Efficient side-chain and backbone assignment in large proteins: application to tGCN5. J Biomol NMR 15:227–239
Liu A, Riek R, Wider G, von Schroetter C, Zahn R, Wüthrich K (2000) NMR experiments for resonance assignments of 13C, 15N doubly-labeled flexible polypeptides: application to the human prion protein hPrP(23–230). J Biomol NMR 16:127–138
Logan TM, Olejniczak ET, Xu RX, Fesik SW (1993) A general method for assigning NMR spectra of denatured proteins using 3D HC(CO)NH-TOCSY triple resonance experiments. J Biomol NMR 3:225–231
Lyons BA, Tashiro M, Cedergren L, Nilsson B, Montelione GT (1993) An improved strategy for determining resonance assignments for isotopically enriched proteins and its application to an engineered domain of staphylococcal protein A. Biochemistry 32:7839–7845
Marintchev A, Frueh D, Wagner G (2007) NMR methods for studying protein-protein interactions involved in translation initiation. Meth Enzymol 430:283–331
Marion D, Ikura M, Tschudin R, Bax A (1989) Rapid recording of 2D NMR spectra without phase cycling. Application to the study of hydrogen exchange in proteins. J Magn Reson 85:393–399
Morris GA, Freeman R (1979) Enhancement of nuclear magnetic resonance signals by polarization transfer. J Am Chem Soc 101:760–762
Muhandiram DR, Kay LE (1994) Gradient-enhanced triple-resonance three-dimensional NMR experiments with improved sensitivity. J Magn Reson B 103:203–216
Rao A, Luo C, Hogan PG (1997) Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol 15:707–747
Ruaro EM, Collavin L, Del Sal G, Haffner R, Oren M, Levine AJ, Schneider C (1997) A proline-rich motif in p53 is required for transactivation-independent growth arrest as induced by Gas1. Proc Natl Acad Sci USA 94:4675–4680
Santoro J, King GC (1992) A constant-time 2D overbodenhausen experiment for inverse correlation of isotopically enriched species. J Magn Reson 97:202–207
Shaka AJ, Keeler J, Frenkiel T, Freeman R (1983) An improved sequence for broadband decoupling: WALTZ-16. J Magn Reson 52:335–338
Shaka AJ, Barker PB, Freeman R (1985) Computer-optimized decoupling scheme for wideband applications and low-level operation. J Magn Reson 64:547–552
Sun ZY, Frueh DP, Selenko P, Hoch JC, Wagner G (2005) Fast assignment of 15N-HSQC peaks using high-resolution 3D HNcocaNH experiments with non-uniform sampling. J Biomol NMR 33:43–50
Takeuchi K, Sun ZY, Wagner G (2008) Alternate 13C-12C labeling for complete mainchain resonance assignments using Cα direct-detection with applicability toward fast relaxing protein systems. J Am Chem Soc 130:17210–17211
Takeuchi K, Frueh D, Sun Z, Hiller S, Wagner G (2010) CACA-TOCSY with alternate 13C-12C labeling: a 13Cα direct detection experiment for mainchain resonance assignment, dihedral angle information, and amino acid type identification. J Biomol NMR 47:55–63
Venot C, Maratrat M, Dureuil C, Conseiller E, Bracco L, Debussche L (1998) The requirement for the p53 proline-rich functional domain for mediation of apoptosis is correlated with specific PIG3 gene transactivation and with transcriptional repression. EMBO J 17:4668–4679
Vuister GW, Bax A (1992) Resolution enhancement and spectral editing of uniformly 13C-enriched proteins by homonuclear broadband 13C decoupling. J Magn Reson 98:428–435
Acknowledgments
This work was supported by the NIH (grants AI37581, GM47467 and EB 002026). M.G would like to thank the Human Frontier science Program (HFSP) for a postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takeuchi, K., Gal, M., Takahashi, H. et al. HNCA-TOCSY-CANH experiments with alternate 13C-12C labeling: a set of 3D experiment with unique supra-sequential information for mainchain resonance assignment. J Biomol NMR 49, 17–26 (2011). https://doi.org/10.1007/s10858-010-9456-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-010-9456-2