Abstract
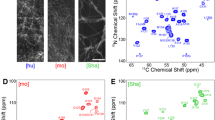
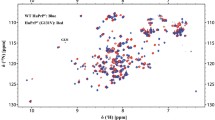
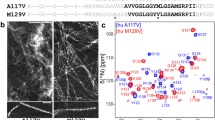
The partial 15N and 13C solid-state NMR resonance assignment of the HET-s prion protein fragment 218–289 in its amyloid form is presented. It is based on experiments measured at MAS frequencies in the range of 20–40 kHz using exclusively adiabatic polarization-transfer schemes. The resonance assignment within each residue is based on two-dimensional 13C––13C correlation spectra utilizing the DREAM mixing scheme. The sequential linking of the assigned residues used a set of two- and three-dimensional 15N––13C correlation experiments. Almost all cross peaks visible in the spectra are assigned, but only resonances from 43 of the 78 amino-acid residues could be detected. The missing residues are thought to be highly disordered and/or highly dynamic giving rise to broad resonance lines that escaped detection in the experiments applied. The line widths of the observed resonances are narrow and comparable to line widths observed in micro-crystalline samples. The 43 assigned residues are located in two fragments of about 20 residues.
Similar content being viewed by others
Abbreviations
- 2D:
-
two-dimensional
- 3D:
-
three-dimensional
- APHH:
-
adiabatic-passage Hartmann-Hahn
- CP:
-
cross polarization
- CW:
-
continuous wave
- DREAM:
-
dipolar recoupling enhanced by amplitude modulation
- HORROR:
-
homonuclear rotary resonance
- MAS:
-
magic angle spinning
- NMR:
-
nuclear magnetic resonance
- NOE:
-
nuclear overhauser effect
- PDSD:
-
proton-driven spin diffusion
- R2T:
-
rotational resonance tickling
- RFDR:
-
radio-frequency driven spin diffusion
- TPPI:
-
time-proportional phase increment
- TRIS:
-
tris(hydroxymethyl)aminomethane
- XiX:
-
X inverse X
References
M. Baldus D.G. Geurts S. Hediger B.H. Meier (1996) J. Magn. Reson. Ser. A 118 140–144 Occurrence Handle10.1006/jmra.1996.0022
M. Baldus B.H. Meier (1996) J. Magn. Reson. Ser. A 121 65–69 Occurrence Handle10.1006/jmra.1996.0137
M. Baldus A.T. Petkova J. Herzfeld R.G. Griffin (1998) Mol. Phys. 95 1197–1207 Occurrence Handle10.1080/002689798166215
A. Balguerie S. Dos Reis C. Ritter S. Chaignepain B. Coulary-Salin V. Forge K. Bathany I. Lascu J.-M. Schmitter R. Riek S.J. Saupe (2003) EMBO J. 22 2071–2081 Occurrence Handle10.1093/emboj/cdg213
A.E. Bennett J.H. Ok R.G. Griffin S. Vega (1992) J. Chem. Phys. 96 8624–8627 Occurrence Handle1992JChPh..96.8624B
A. Bockmann A. Lange A. Galinier S. Luca N. Giraud M. Juy H. Heise R. Montserret F. Penin M. Baldus (2003) J. Biomol. NMR 27 323–339
A. Brinkmann M. Eden M.H. Levitt (2000) J. Chem. Phys. 112 8539–8554 Occurrence Handle10.1063/1.481458 Occurrence Handle2000JChPh.112.8539B
M. Carravetta M. Eden X. Zhao A. Brinkmann M.H. Levitt (2000) Chem. Phys. Lett. 321 205–215 Occurrence Handle10.1016/S0009-2614(00)00340-7
F. Castellani B. Rossum Particlevan A. Diehl M. Schubert K. Rehbein H. Oschkinat (2002) Nature 420 98–102 Occurrence Handle10.1038/nature01070 Occurrence Handle2002Natur.420...98C
F. Castellani B.J. Rossum Particlevan A. Diehl K. Rehbein H. Oschkinat (2003) Biochemistry 42 11476–11483 Occurrence Handle10.1021/bi034903r
P.R. Costa B.Q. Sun R.G. Griffin (1997) J. Am. Chem. Soc. 119 10821–10830
V. Coustou-Linares M.-L. Maddelein J. Begueret S.J. Saupe (2001) Mol. Microbiol. 42 1325–1335 Occurrence Handle10.1046/j.1365-2958.2001.02707.x
A. Detken E.H. Hardy M. Ernst M. Kainosho T. Kawakami S. Aimoto B.H. Meier (2001) J. Biomol. NMR 20 203–221 Occurrence Handle10.1023/A:1011212100630
A. Detken E.H. Hardy M. Ernst B.H. Meier (2002) Chem. Phys. Lett. 356 298–304 Occurrence Handle10.1016/S0009-2614(02)00335-4
S. Dos Reis B. Coulary-Salin V. Forge I. Lascu J. Begueret S.J. Saupe (2002) J. Biol. Chem. 277 5703–5706 Occurrence Handle10.1074/jbc.M110183200
W.L. Earl D.L. VanDerHart (1982) J. Magn. Reson. 48 35–54
M. Ernst A. Detken A. Bockmann B.H. Meier (2003) J. Am. Chem. Soc. 125 15807–15810 Occurrence Handle10.1021/ja0369966
A.J.v. Gammeren F.B. Hulsbergen J.G. Hollander H.J.M.d. Groot (2005) J. Biomol. NMR 31 279–293 Occurrence Handle10.1007/s10858-005-1604-8
N.L. Glass D. Jacobson P. Shiu (1997) Annu. Rev. Genet. 34 165–186
R.K. Harris E.D. Becker S.M. Cabral de Menezes R. Goodfellow P. Granger (2002) Magn. Reson. Chem. 40 489–505
S. Hediger B.H. Meier R.R. Ernst (1995) Chem. Phys. Lett. 240 449 Occurrence Handle10.1016/0009-2614(95)00505-X
S. Hediger B.H. Meier N.D. Kurur G. Bodenhausen R.R. Ernst (1994) Chem. Phys. Lett. 223 283–288 Occurrence Handle10.1016/0009-2614(94)00470-6
M. Hong (1999) J. Biomol. NMR 15 1–14 Occurrence Handle10.1023/A:1008334204412
T.I. Igumenova A.E. McDermott K.W. Zilm R.W. Martin E.K. Paulson A.J. Wand (2004) J. Am. Chem. Soc. 126 6720–6727
T.I. Igumenova A.J. Wand A.E. McDermott (2004) J. Am. Chem. Soc. 126 5323–5331
Y. Ishii R. Tycko (2000) J. Am. Chem. Soc. 122 1443–1455
C.P. Jaroniec C.E. MacPhee V.S. Bajaj M.T. McMahon C.M. Dobson R.G. Griffin (2004) PNAS 101 711–716 Occurrence Handle10.1073/pnas.0304849101 Occurrence Handle2004PNAS..101..711J
R.L.J. Keller (2004) The Computer Aided Resonance Assignment Tutorial Cantina Verlag Goldau
A. Lange S. Becker K. Seidel K. Giller O. Pongs M. Baldus (2005) Angew. Chem.-Int. Edit. 44 2089–2092
M.L. Maddelein S. Dos Reis S. Duvezin-Caubet B. Coulary-Salin S.J. Saupe (2002) Proc. Natl. Acad. Sci. U.S.A 99 7402–7407 Occurrence Handle10.1073/pnas.072199199 Occurrence Handle2002PNAS...99.7402M
Marion, D. and Wuthrich, K. (1983) Biochem. Biophys. Res. Commun., 967–974
C.R. Morcombe K.W. Zilm (2003) J. Magn. Reson. 162 479–486 Occurrence Handle10.1016/S1090-7807(03)00082-X Occurrence Handle2003JMagR.162..479M
J. Pauli M. Baldus B. Rossum Particlevan H. Groot Particlede H. Oschkinat (2001) Chembiochem 2 272–281 Occurrence Handle10.1002/1439-7633(20010401)2:4<272::AID-CBIC272>3.0.CO;2-2
A.T. Petkova M. Baldus M. Belenky M. Hong R.G. Griffin J. Herzfeld (2003) J. Magn. Reson. 160 1–12 Occurrence Handle10.1016/S1090-7807(02)00137-4 Occurrence Handle2003JMagR.160....1P
A.T. Petkova G. Buntkowsky F. Dyda R.D. Leapman W.M. Yau R. Tycko (2004) J. Mol. Biol. 335 247–260 Occurrence Handle10.1016/j.jmb.2003.10.044
C.M. Rienstra M. Hohwy M. Hong R.G. Griffin (2000) J. Am. Chem. Soc. 122 10979–10990 Occurrence Handle10.1021/ja001092v
C. Ritter M.L. Maddelein A.B. Siemer T. Luhrs M. Ernst B.H. Meier S.J. Saupe R. Riek (2005) Nature 435 844–848 Occurrence Handle10.1038/nature03793 Occurrence Handle2005Natur.435..844R
Samoson, A., Tuherm, T., Past, J., Reinhold, A., Anupold, T. and Heinmaa N. (2005) In New Techniques in Solid-State NMR, Vol. 246, pp. 15–31
S.J. Saupe (2000) Microbiol. Mol. Biol. Rev. 64 489–502
S.K. Straus T. Bremi R.R. Ernst (1998) J. Biomol. NMR 12 39–50 Occurrence Handle10.1023/A:1008280716360
K. Takegoshi K. Nomura T. Terao (1995) Chem. Phys. Lett. 232 424–428 Occurrence Handle10.1016/0009-2614(94)01399-G
K. Takegoshi K. Nomura T. Terao (1997) J. Magn. Reson. 127 206–216 Occurrence Handle10.1006/jmre.1997.1191
R. Tycko (2004) Curr. Opin. Struct. Biol. 14 96–103 Occurrence Handle10.1016/j.sbi.2003.12.002
B.J. Rossum Particlevan F. Castellani J. Pauli K. Rehbein J. Hollander H.J.M. Groot Particlede H. Oschkinat (2003) J. Biomol. NMR 25 217–223
R. Verel M. Baldus M. Ernst B.H. Meier (1998) Chem. Phys. Lett. 287 421–428 Occurrence Handle10.1016/S0009-2614(98)00172-9
R. Verel M. Ernst B.H. Meier (2001) J. Magn. Reson. 150 81–99 Occurrence Handle10.1006/jmre.2001.2310 Occurrence Handle2001JMagR.150...81V
S.G. Zech A.J. Wand A.E. McDermott (2005) J. Am. Chem. Soc. 127 8618–8626 Occurrence Handle10.1021/ja0503128
Author information
Authors and Affiliations
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Siemer, A.B., Ritter, C., Steinmetz, M.O. et al. 13C, 15N Resonance Assignment of Parts of the HET-s Prion Protein in its Amyloid Form. J Biomol NMR 34, 75–87 (2006). https://doi.org/10.1007/s10858-005-5582-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10858-005-5582-7