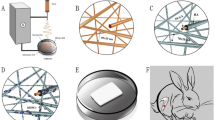
Abstract
Bioactive glasses (BG) are known for their ability to bond to bone tissue. However, in critical situations, even the osteogenic properties of BG may be not enough to induce bone consolidation. Thus, the enrichment of BG with polymers such as Poly (D, L-lactic-co-glycolic) acid (PLGA) and associated to photobiomodulation (PBM) may be a promising strategy to promote bone tissue healing. The aim of the present study was to investigate the in vivo performance of PLGA supplemented BG, associated to PBM therapy, using an experimental model of cranial bone defect in rats. Rats were distributed in 4 different groups (Bioglass, Bioglass/PBM, Bioglas/PLGA and BG/PLGA/PBM). After the surgical procedure to induce cranial bone defects, the pre-set samples were implanted and PBM treatment (low-level laser therapy) started (808 nm, 100 mW, 30 J/cm2). After 2 and 6 weeks, animals were euthanized, and the samples were retrieved for the histopathological, histomorphometric, picrosirius red staining and immunohistochemistry analysis. At 2 weeks post-surgery, it was observed granulation tissue and areas of newly formed bone in all experimental groups. At 6 weeks post-surgery, BG/PLGA (with or without PBM) more mature tissue around the biomaterial particles. Furthermore, there was a higher deposition of collagen for BG/PLGA in comparison with BG/PLGA/PBM, at second time-point. Histomorphometric analysis demonstrated higher values of BM.V/TV for BG compared to BG/PLGA (2 weeks post-surgery) and N.Ob/T.Ar for BG/PLGA compared to BG and BG/PBM (6 weeks post-surgery). This current study concluded that the use of BG/PLGA composites, associated or not to PBM, is a promising strategy for bone tissue engineering.










Similar content being viewed by others
References
Akter F. Tissue engineering made easy. London, UK: Academic Press; 2016.
Patel S, Caldwell JM, Doty SB, Levine WN, Rodeo S, Soslowsky LJ, et al. Integrating soft and hard tissues via interface tissue engineering. J Orthop Res: Off Publ Orthop Res Soc. 2017. https://doi.org/10.1002/jor.23810.
Hench LL, Polak JM. Third-generation biomedical materials. Science 2002;295:1014. https://doi.org/10.1126/science.1067404.
Fernandes KR, Magri AMP, Kido HW, Parisi JR, Assis L, Fernandes KPS, et al. Biosilicate/PLGA osteogenic effects modulated by laser therapy: In vitro and in vivo studies. J Photochemistry Photobiol B Biol. 2017;173:258. https://doi.org/10.1016/j.jphotobiol.2017.06.002.
Felix Lanao RP, Bosco R, Leeuwenburgh SC, Kersten-Niessen MJ, Wolke JG, van den Beucken JJ, et al. RANKL delivery from calcium phosphate containing PLGA microspheres. J Biomed Mater Res Part A. 2013;101:3123. https://doi.org/10.1002/jbm.a.34623.
Felix Lanao RP, Leeuwenburgh SC, Wolke JG, Jansen JA. In vitro degradation rate of apatitic calcium phosphate cement with incorporated PLGA microspheres. Acta Biomaterialia. 2011;7:3459. https://doi.org/10.1016/j.actbio.2011.05.036.
Hench LL. The story of Bioglass. J Mater Sci Mater Med. 2006;17:967. https://doi.org/10.1007/s10856-006-0432-z.
Gerhardt LC, Boccaccini AR. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010;3:3867. https://doi.org/10.3390/ma3073867.
Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochemical Biophysical Res Commun. 2000;276:461. https://doi.org/10.1006/bbrc.2000.3503.
Navarro M, Michiardi A, Castano O, Planell JA. Biomaterials in orthopaedics. J R Soc Interface 2008;5:1137. https://doi.org/10.1098/rsif.2008.0151.
Fernandes KR, Magri AMP, Kido HW, Ueno F, Assis L, Fernandes KPS. et al.Characterization and biological evaluation of the introduction of PLGA into biosilicate(R).J Biomed Mater Res. Part B. Appl Biomater. 2017;105:1063. https://doi.org/10.1002/jbm.b.33654.
Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005;26:5474. https://doi.org/10.1016/j.biomaterials.2005.02.002.
Day RM. Bioactive glass stimulates the secretion of angiogenic growth factors and angiogenesis in vitro. Tissue Eng. 2005;11:768. https://doi.org/10.1089/ten.2005.11.768.
Coelho RC, Zerbinati LP, de Oliveira MG, Weber JB. Systemic effects of LLLT on bone repair around PLLA-PGA screws in the rabbit tibia. Lasers Med Sci. 2014;29:703. https://doi.org/10.1007/s10103-013-1384-4.
Pinheiro AL, Limeira Junior Fde A, Gerbi ME, Ramalho LM, Marzola C, Ponzi EA, et al. Effect of 830-nm laser light on the repair of bone defects grafted with inorganic bovine bone and decalcified cortical osseus membrane. J Clin Laser Med Surg. 2003;21:301. https://doi.org/10.1089/104454703322564523.
Barbos Pinheiro AL, Limeira Junior FA, Marquez Gerbi ME, Pedreira Ramalho LM, Marzola C, Carneiro Ponzi EA, et al. Effect of 830-nm laser light on the repair of bone defects grafted with inorganic bovine bone and decalcified cortical osseous membrane. J Clin Laser Med Surg. 2003;21:383. https://doi.org/10.1089/104454703322650202.
Huang YY, Sharma SK, Carroll J, Hamblin MR. Biphasic dose response in low level light therapy - an update. Dose-response : a Publ Int Hormesis Soc. 2011;9:602. https://doi.org/10.2203/dose-response.11-009.Hamblin.
Renno AC, McDonnell PA, Parizotto NA, Laakso EL. The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro. Photomed Laser Surg. 2007;25:275. https://doi.org/10.1089/pho.2007.2055.
Assis L, Moretti AI, Abrahao TB, de Souza HP, Hamblin MR, Parizotto NA. Low-level laser therapy (808 nm) contributes to muscle regeneration and prevents fibrosis in rat tibialis anterior muscle after cryolesion. Lasers Med Sci. 2013;28:947. https://doi.org/10.1007/s10103-012-1183-3.
Barbosa D, de Souza RA, Xavier M, da Silva FF, Arisawa EA, Villaverde AG. Effects of low-level laser therapy (LLLT) on bone repair in rats: optical densitometry analysis. Lasers Med Sci. 2013;28:651. https://doi.org/10.1007/s10103-012-1125-0.
Pires Oliveira DA, de Oliveira RF, Zangaro RA, Soares CP. Evaluation of low-level laser therapy of osteoblastic cells. Photomed Laser Surg. 2008;26:401. https://doi.org/10.1089/pho.2007.2101.
Favaro-Pipi E, Feitosa SM, Ribeiro DA, Bossini P, Oliveira P, Parizotto NA, et al. Comparative study of the effects of low-intensity pulsed ultrasound and low-level laser therapy on bone defects in tibias of rats. Lasers Med Sci. 2010;25:727. https://doi.org/10.1007/s10103-010-0772-2.
Bossini PS, Renno AC, Ribeiro DA, Fangel R, Ribeiro AC, Lahoz Mde A, et al. Low level laser therapy (830 nm) improves bone repair in osteoporotic rats: similar outcomes at two different dosages. Exp Gerontol. 2012;47:136. https://doi.org/10.1016/j.exger.2011.11.005.
Renno AC, McDonnell PA, Crovace MC, Zanotto ED, Laakso L. Effect of 830 nm laser phototherapy on osteoblasts grown in vitro on Biosilicate scaffolds. Photomed Laser Surg. 2010;28:131. https://doi.org/10.1089/pho.2009.2487.
Pinto KN, Tim CR, Crovace MC, Matsumoto MA, Parizotto NA, Zanotto ED, et al. Effects of biosilicate((R)) scaffolds and low-level laser therapy on the process of bone healing. Photomed Laser Surg. 2013;31:252. https://doi.org/10.1089/pho.2012.3435.
Luvizuto ER, Queiroz TP, Margonar R, Panzarini SR, Hochuli-Vieira E, Okamoto T, et al. Osteoconductive properties of beta-tricalcium phosphate matrix, polylactic and polyglycolic acid gel, and calcium phosphate cement in bone defects. J Craniofacial Surg. 2012;23:e430. https://doi.org/10.1097/SCS.0b013e31825e4abf.
Kubota T, Hasuike A, Ozawa Y, Yamamoto T, Tsunori K, Yamada Y, et al. Regenerative capacity of augmented bone in rat calvarial guided bone augmentation model. J Periodontal Implant Sci. 2017;47:77. https://doi.org/10.5051/jpis.2017.47.2.77.
Magri AM, Fernandes KR, Assis L, Mendes NA, da Silva Santos AL, de Oliveira, Dantas E, et al. Photobiomodulation and bone healing in diabetic rats: evaluation of bone response using a tibial defect experimental model. Lasers Med Sci. 2015;30:1949. https://doi.org/10.1007/s10103-015-1789-3.
Magri AMP, Fernandes KR, Ueno FR, Kido HW, Da Silva AC, Braga FJC, et al. Osteoconductive properties of two different bioactive glass forms (powder and fiber) combined with collagen. Appl Surf Sci. 2017;423:557.
Andrade GB, Montes G, Conceição G, Saldiva PHN. Use of the Picrosirius-polarization method to age fibrotic lesions in the hepatic granulomas produced in experimental murine schistosomiasis. Pathog Glob Health 1999;93:265. https://doi.org/10.1080/00034989958528.
Garavello-Freitas I, Baranauskas V, Joazeiro PP, Padovani CR, Dal Pai-Silva M, da Cruz-Hofling MA. Low-power laser irradiation improves histomorphometrical parameters and bone matrix organization during tibia wound healing in rats. J Photochemistry Photobiol B, Biol. 2003;70:81.
Bossini PS, Renno AC, Ribeiro DA, Fangel R, Peitl O, Zanotto ED, et al. Biosilicate(R) and low-level laser therapy improve bone repair in osteoporotic rats. J Tissue Eng Regenerative Med. 2011;5:229. https://doi.org/10.1002/term.309.
Tim CR, Pinto KN, Rossi BR, Fernandes K, Matsumoto MA, Parizotto NA, et al. Low-level laser therapy enhances the expression of osteogenic factors during bone repair in rats. Lasers Med Sci. 2014;29:147. https://doi.org/10.1007/s10103-013-1302-9.
O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today 2011;14:88. https://doi.org/10.1016/S1369-7021(11)70058-X.
Day RM, Boccaccini AR, Shurey S, Roether JA, Forbes A, Hench LL, et al. Assessment of polyglycolic acid mesh and bioactive glass for soft-tissue engineering scaffolds. Biomaterials 2004;25:5857. https://doi.org/10.1016/j.biomaterials.2004.01.043.
Filipowska J, Pawlik J, Cholewa-Kowalska K, Tylko G, Pamula E, Niedzwiedzki L, et al. Incorporation of sol-gel bioactive glass into PLGA improves mechanical properties and bioactivity of composite scaffolds and results in their osteoinductive properties. Biomed Mater. 2014;9:065001. https://doi.org/10.1088/1748-6041/9/6/065001.
Pamula E, Kokoszka J, Cholewa-Kowalska K, Laczka M, Kantor L, Niedzwiedzki L, et al. Degradation, bioactivity, and osteogenic potential of composites made of PLGA and two different sol-gel bioactive glasses. Ann Biomed Eng. 2011;39:2114. https://doi.org/10.1007/s10439-011-0307-4.
Sarvestani FK, Dehno NS, Nazhvani SD, Bagheri MH, Abbasi S, Khademolhosseini Y, et al. Effect of low-level laser therapy on fracture healing in rabbits. Laser Ther. 2017;26:189. https://doi.org/10.5978/islsm.17-OR-14.
Tim CR, Bossini PS, Kido HW, Malavazi I, von Zeska Kress MR, Carazzolle MF, et al. Effects of low-level laser therapy on the expression of osteogenic genes during the initial stages of bone healing in rats: a microarray analysis. Lasers Med Sci. 2015;30:2325. https://doi.org/10.1007/s10103-015-1807-5.
Oliveira P, Fernandes KR, Sperandio EF, Pastor FA, Nonaka KO, Parizotto NA, et al. Comparative study of the effects of low-level laser and low-intensity ultrasound associated with biosilicate((r)) on the process of bone repair in the rat Tibia. Rev Brasileira De Ortop. 2012;47:102. https://doi.org/10.1016/S2255-4971(15)30352-9.
Pinheiro AL, Santos NR, Oliveira PC, Aciole GT, Ramos TA, Gonzalez TA, et al. The efficacy of the use of IR laser phototherapy associated to biphasic ceramic graft and guided bone regeneration on surgical fractures treated with miniplates: a Raman spectral study on rabbits. Lasers Med Sci. 2013;28:513. https://doi.org/10.1007/s10103-012-1096-1.
Renno AC, Nejadnik MR, van de Watering FC, Crovace MC, Zanotto ED, Hoefnagels JP, et al. Incorporation of bioactive glass in calcium phosphate cement: material characterization and in vitro degradation. J Biomed Mater Res Part A. 2013;101:2365. https://doi.org/10.1002/jbm.a.34531.
Renno AC, van de Watering FC, Nejadnik MR, Crovace MC, Zanotto ED, Wolke JG, et al. Incorporation of bioactive glass in calcium phosphate cement: An evaluation. Acta Biomaterialia. 2013;9:5728. https://doi.org/10.1016/j.actbio.2012.11.009.
Oh JH, Kim HJ, Kim TI, Woo KM. Comparative evaluation of the biological properties of fibrin for bone regeneration. BMB Rep. 2014;47:110.
Marsell R, Einhorn TA. The biology of fracture healing. Injury 2011;42:551. https://doi.org/10.1016/j.injury.2011.03.031.
Rizwan M, Hamdi M, Basirun WJ. Bioglass(R) 45S5-based composites for bone tissue engineering and functional applications. J Biomed Mater Res Part A. 2017;105:3197. https://doi.org/10.1002/jbm.a.36156.
Skondra FG, Koletsi D, Eliades T, Farmakis ETR. The effect of low-level laser therapy on bone healing after rapid maxillary expansion: a systematic review. Photomed Laser Surg. 2018;36:61. https://doi.org/10.1089/pho.2017.4278.
Santinoni CD, Oliveira HF, Batista VE, Lemos CA, Verri FR. Influence of low-level laser therapy on the healing of human bone maxillofacial defects: A systematic review. J Photochemistry Photobiol B Biol. 2017;169:83. https://doi.org/10.1016/j.jphotobiol.2017.03.004.
Noba C, Mello-Moura ACV, Gimenez T, Tedesco TK, Moura-Netto C. Laser for bone healing after oral surgery: systematic review. Lasers Med Sci. 2018;33:667. https://doi.org/10.1007/s10103-017-2400-x.
Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose-response: A Publ Int Hormesis Soc. 2009;7:358. https://doi.org/10.2203/dose-response.09-027.Hamblin.
Oliveira P, Ribeiro DA, Pipi EF, Driusso P, Parizotto NA, Renno AC. Low level laser therapy does not modulate the outcomes of a highly bioactive glassceramic (Biosilicate) on bone consolidation in rats. J Mater Sci Mater Med. 2010;21:1379. https://doi.org/10.1007/s10856-009-3945-4.
Li G, Hu J, Chen H, Chen L, Zhang N, Zhao L, et al. Enamel matrix derivative enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells on the titanium implant surface. Organogenesis 2017;13:103. https://doi.org/10.1080/15476278.2017.1331196.
Hienz SA, Paliwal S, Ivanovski S. Mechanisms of bone resorption in periodontitis. J Immunol Res. 2015;2015:615486. https://doi.org/10.1155/2015/615486.
de Vernejoul MC. Sclerosing bone disorders. Best practice & research. Clin Rheumatol. 2008;22:71. https://doi.org/10.1016/j.berh.2007.12.011.
Acknowledgements
The authors would like to acknowledge funding agencies FAPESP (grant number: 2014/20546-0) and CNPq for the financial support of this research and CAPES for scholarship to AMPM. In addition, the authors would like to thank Dr Ingrid Regina Avanzi for helping during some euthanasia of the animals and Prof. Dr Flavia de Oliveira and Hananiah Tardivo Quintana for assistance with picrosirius analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Magri, A.M.P., Fernandes, K.R., Kido, H.W. et al. Bioglass/PLGA associated to photobiomodulation: effects on the healing process in an experimental model of calvarial bone defect. J Mater Sci: Mater Med 30, 105 (2019). https://doi.org/10.1007/s10856-019-6307-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-019-6307-x