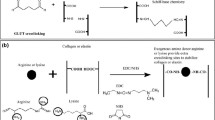
Abstract
Glutaraldehyde (GLUT) crosslinked bioprosthetic heart valves (BHVs) might fail due to progressive degradation and calcification. GLUT cannot stabilize glycosaminoglycans (GAGs), which are important for BHVs’ life time. In this current study we developed a new BHVs preparation strategy using exogenous hyaluronic acid (HA)/chondroitin sulfate (CS) supplement and sodium trimetaphosphate (STP) crosslinking method. Exogenous HA and CS provide additional GAGs for pericardiums. STP could link two GAGs by reacting with hydroxyl groups in GAGs’ repeating polysaccharides units. The feeding ratios of HA/CS were optimized. The GAGs content and long-term stability in vitro, biocompatibility, the in vivo GAGs stability and anti-calcification potential of GLUT/HA/CS and STP treated pericardiums were characterized. We demonstrated that GLUT/HA/CS and STP treated pericardiums had sufficiently increased GAGs’ amount and stability and decreased calcification. This new exogenous hyaluronic acid/chondroitin sulfate supplement and sodium trimetaphosphate crosslinking strategy would be a promising method to make BHVs with better structural stability and anti-calcification properties.







Similar content being viewed by others
References
Manji RA, et al. Porcine bioprosthetic heart valves: the next generation. Am Heart J. 2012;164(2):177–85.
Rapoport HS, et al. Mechanisms of the in vivo inhibition of calcification of bioprosthetic porcine aortic valve cusps and aortic wall with triglycidylamine/mercapto bisphosphonate. Biomaterials. 2007;28(4):690–9.
Hedayat M, Asgharzadeh H, Borazjani I. Platelet activation of mechanical versus bioprosthetic heart valves during systole. J Biomech. 2017;56:111–6.
Human P, Zilla P. The possible role of immune responses in bioprosthetic heart valve failure. J Heart Valve Dis. 2001;10(4):460.
Zilla P, et al. Prosthetic heart valves: catering for the few. Biomaterials. 2008;29(4):385–406.
Vyavahare N, et al. Mechanisms of bioprosthetic heart valve failure: fatigue causes collagen denaturation and glycosaminoglycan loss. J Biomed Mater Res. 1999;46(1):44.
Tam H, et al. A novel crosslinking method for improved tear resistance andbiocompatibility of tissue based biomaterials. Biomaterials. 2015;66:83–91.
Leong J, et al. Neomycin and carbodiimide crosslinking as an alternative to glutaraldehyde for enhanced durability of bioprosthetic heart valves. J Biomater Appl. 2013;27(8):948.
Levy RJ, et al. Calcification of subcutaneously implanted type I collagen sponges. Effects of formaldehyde and glutaraldehyde pretreatments. Am J Pathol. 1986;122(1):71–82.
Golomb G, et al. The role of glutaraldehyde-induced cross-links in calcification of bovine pericardium used in cardiac-valve bioprostheses. Am J Pathol. 1987;127(1):122–30.
Bezuidenhout D, et al. The effects of cross-link density and chemistry on the calcification potential of diamine-extended glutaraldehyde-fixed bioprosthetic heart-valve materials. Biotechnol Appl Biochem. 2009;54:133–40.
Jorge-Herrero E, et al. Biocompatibility and calcification of bovine pericardium employed for the construction of cardiac bioprostheses treated with different chemical crosslink methods. Artif Organs. 2010;34(5):168–76.
Tripi DR, Vyavahare NR. Neomycin and pentagalloyl glucose enhanced cross-linking for elastin and glycosaminoglycans preservation in bioprosthetic heart valves. J Biomater Appl. 2014;28(28):757–66.
Raghavan D, Simionescu DT, Vyavahare NR. Neomycin prevents enzyme-mediated glycosaminoglycan degradation in bioprosthetic heart valves. Biomaterials. 2007;28(18):2861.
Raghavan D, Starcher BC, Vyavahare NR. Neomycin binding preserves extracellular matrix in bioprosthetic heart valves during in vitro cyclic fatigue and storage. Acta Biomater. 2009;5(4):983–92.
Shah SR, Vyavahare NR. The effect of glycosaminoglycan stabilization on tissue buckling in bioprosthetic heart valves. Biomaterials. 2008;29(11):1645–53.
Tripi DR, Vyavahare NR. Neomycin and pentagalloyl glucose enhanced cross-linking for elastin and glycosaminoglycans preservation in bioprosthetic heart valves. J Biomater Appl. 2014;28(5):757–66.
Lovekamp J, Vyavahare N. Periodate‐mediated glycosaminoglycan stabilization in bioprosthetic heart valves. J Biomed Mater Res. 2001;56(4):478–86.
Mercuri JJ, Lovekamp JJ. Glycosaminoglycan-targeted fixation for improved bioprosthetic heart valve stabilization. Biomaterials. 2007;28(26):496–503.
Gao F, et al. Preparation and characterization of starch crosslinked with sodium trimetaphosphate and hydrolyzed by enzymes. Carbohydr Polym. 2014;103(1):310–8.
Carbinatto FM, et al. Physical properties of pectin-high amylose starch mixtures cross-linked with sodium trimetaphosphate. Int J Pharm. 2012;423(2):281.
Scott JE, Haigh M. Proteoglycan-type I collagen fibril interactions in bone and non-calcifying connective tissues. Biosci Rep. 1985;5(1):71–81.
Lowther DA, Preston BN, Meyer FA. Isolation and properties of chondroitin sulphates from bovine heart valves. Biochem J. 1970;118(4):595–601.
Caballero A, et al. Evaluation of transcatheter heart valve biomaterials: biomechanical characterization of bovine and porcine pericardium. J Mech Behav Biomed Mater. 2017;75:486–94.
Lovekamp JJ, et al. Stability and function of glycosaminoglycans in porcine bioprosthetic heart valves. Biomaterials. 2006;27(8):1507–18.
Tam H. et al. Fixation of bovine pericardium-based tissue biomaterial with irreversible chemistry improves biochemical and biomechanical properties. J Cardiovasc Transl Res. 2017;10(2):194–205. https://doi.org/10.1007/s12265-017-9733-5.
Jiang BPD, et al. Targeting heparin to collagen within extracellular matrix significantly reduces thrombogenicity and improves endothelialization of decellularized tissues. Biomacromolecules. 2016;17(12):3940.
Li J, et al. A novel natural hirudin facilitated anti-clotting polylactide membrane via hydrogen bonding interaction. J Membr Sci. 2017;523:505–14.
Cigliano A, et al. Fine structure of glycosaminoglycans from fresh and decellularized porcine cardiac valves and pericardium. Biochem Res Int. 2012;2012:979351.
Jorgeherrero E, et al. Study of the calcification of bovine pericardium: analysis of the implication of lipids and proteoglycans. Biomaterials. 1991;12(7):683–9.
Ohri R, et al. Hyaluronic acid grafting mitigates calcification of glutaraldehyde-fixed bovine pericardium. J Biomed Mater Res A. 2004;70A(2):328–34.
Murata K. Acidic glycosaminoglycans in human heart valves. J Mol Cell Cardiol. 1981;13(3):281–92.
Zhou J, et al. Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials. 2010;31(9):2549–54.
Acknowledgements
This work was supported by National Natural Science Foundation of China (31700833), Young Elite Scientists Sponsorship Program by CAST (2017QNRC001), the Fundamental Research Funds for the Central Universities (YJ201641), National Key Research and Development Programs (2017YFC1104200, 2016YFC1102200), and the Program of Introducing Talents of Discipline to Universities (111 Project, No. B16033). We would like to thank VENUS MEDTECH Inc. (Hangzhou, China) for providing us with fresh pericardiums.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lei, Y., Ning, Q., Tang, Y. et al. Exogenous hyaluronic acid and chondroitin sulfate crosslinking treatment for increasing the amount and stability of glycosaminoglycans in bioprosthetic heart valves. J Mater Sci: Mater Med 30, 38 (2019). https://doi.org/10.1007/s10856-019-6237-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-019-6237-7