Abstract
Anticoagulation therapy plays a vital role in the prevention of blood clot formation during hemodialysis and hemofiltration, especially for critical care patients. Here, we synthesized a novel argatroban (Arg)-modified polysulfone (PSf) membrane for anticoagulation. Arg was grafted onto the PSF membrane via chemical modification to increase membrane hydrophilicity. Protein adsorption, coagulation, as well as activation of platelets and complement systems were greatly reduced on the Arg-modified PSf membrane. Thus, the recalcification time and the activated partial thrombin time (APTT) were increased after the modification. In comparison with the pristine PSf membrane, the Arg-modified PSf membrane showed better hemocompatibility and anticoagulation properties, indicating its potential for applications in hemodialysis and hemofiltration.

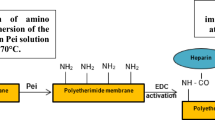
Modification of the PSf membrane has been investigated in attempts to further enhance the anticoagulation properties of the hemodialysis membranes, including a heparin-modified PSf membrane. However, heparin can inhibit plasma-free thrombin, and cause the occurrence of heparin-induced thrombocytopenia (HIT), which increases the risk of bleeding during dialysis in critical care patients. To address this problem, we modified PSf membrane with as a novel direct thrombin inhibitors, argatroban (Arg). It can reversibly bind to thrombin, inhibiting not only the plasma-free thrombin in the blood, but also clot-bound thrombin.















Similar content being viewed by others
References
Hase T, Sirajuddin S, Maluso P, Bangalore R, DePalma L, Sarani B. Platelet dysfunction in critically ill patients. Blood Coagul Fibrinolysis. 2017;28:475–8.
Liu C, Mao Z, Kang H, Hu J, Zhou F. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: a meta-analysis with trial sequential analysis of randomized controlled trials. Crit Care. 2016;20:144.
Hyvärinen S, Meri S, Jokiranta TS. Disturbed sialic acid recognition on endothelial cells and platelets in complement attack causes atypical hemolytic uremic syndrome. Blood. 2016;127:2701–10.
Li H, Cheng W, Liu K, Chen L, Huang Y, Wang X, Lv Z, He J, Li C. Reinforced collagen with oxidized microcrystalline cellulose shows improved hemostatic effects. Carbohydr Polym. 2017;165:30–38.
Major TC, Handa H, Annich GM, Bartlett RH. Development and hemocompatibility testing of nitric oxide releasing polymers using a rabbit model of thrombogenicity. J Biomater Appl. 2014;29:479–501.
Chen Z, Tan L, Hu L, Zhang Y, Wang S, Lv F. Real Colorimetric Thrombin Aptasensor by Masking Surfaces of Catalytically Active Gold Nanoparticles. ACS Appl Mater Interfaces. 2016;8:102–8.
Wiegner R, Chakraborty S, Huber-Lang M. Complement-coagulation crosstalk on cellular and artificial surfaces. Immunobiology. 2016;221:1073–9.
Palygin O, Ilatovskaya DV, Staruschenko A. Protease-activated receptors in kidney disease progression. Am J Physiol Ren Physiol. 2016;311:1140–44.
Zhang Y, Ning J, Veeraragoo P, Li Y, Dai S. Hemodialysis with a dialyzer loaded with argatroban may be performed in vivo without a systemic anticoagulant. Blood Purif. 2012;33:300–6.
Mineshima M. Optimal Design of Dialyzers. Contrib Nephrol. 2017;189:204–9.
Melo NC, Moyses RM, Elias RM, Castro MC. Reprocessing high-flux polysulfone dialyzers does not negatively impact solute removal in short-daily online hemodiafiltration. Hemodial Int. 2014;18:473–80.
Anaya S, Serrano B, Herrero B, Cervera A, Baselga J. γ-Alumina modification with long chain carboxylic acid surface nanocrystals for biocompatible polysulfonenanocomposites. ACS Appl Mater Interfaces. 2014;6:14460–8.
Bowry SK, Gatti E, Vienken J. Contribution of polysulfone membranes to the success of convective dialysis therapies. Contrib Nephrol. 2011;173:110–8.
Bargnoux AS, Cristol JP, Jaussent I, Chalabi L, Bories P, Dion JJ, et al. Vitamin E-coated polysulfone membrane improved red blood cell antioxidant status in hemodialysis patients. J Nephrol. 2013;26:556–63.
Rodríguez-Ribera L, Corredor Z, Silva I, Díaz JM, Ballarín J, Marcos R, Pastor S, Coll E. Vitamin E-coated dialysis membranes reduce the levels of oxidative genetic damage in hemodialysis patients. Mutat Res. 2017;815:16–21.
Kilduff JE, Mattaraj S, Pieracci JP, Belfort G. Photochemical modification of poly(ether sulfone) and sulfonated poly(sulfone) nanofiltration membranes for control of fouling by natural organic matter. Desalination. 2000;132:133–42.
Good K, Escobar I, Xu X, Coleman M, Ponting M. Modification of commercial water treatment membranes by ion beam irradiation. Desalination. 2002;146:259–64.
Mahlicli FY, Altinkaya SA. Surface modification of polysulfone based hemodialysis membranes with layer by layer selfassembly of polyethyleneimine/alginate-heparin:a simple polyelectrolyte blend approach forheparin immobilization. J Mater Sci Mater Med. 2013;24:533–46.
Xie B, Zhang R, Zhang H, Xu A, Deng Y, Lv Y, Deng F, Wei S. Decoration of heparin and bovine serum albumin on polysulfone membrane assisted via polydopamine strategy for hemodialysis. J Biomater Sci Polym Ed. 2016;27:880–97.
Brand B, Graf L. New anticoagulants—direct thrombin inhibitors. Ther Umsch. 2012;69:643–9.
Greinacher A. Clinical practice. Heparin-Induced Thrombocytopenia. N Engl J Med. 2015;373:252–61.
McKenzie SE, Sachais BS. Advances in the pathophysiology and treatment of heparin-induced thrombocytopenia. Curr Opin Hematol. 2014;21:380–7.
Lovecchio F. Heparin-induced thrombocytopenia. Clin Toxicol (Phila). 2014;52:579–83.
Kodras K, Benesch T, Neumann I, Haas M. Comparison of two dialysers (AN69ST vs. FX100)for heparin-free dialysis in patients with oralanticoagulation. Blood Purif. 2008;26:226–30.
Sagedal S, Witczak BJ, Osnes K, Hartmann A, Os I, Eikvar L, Klingenberg O, Brosstad F. A heparin-coated dialysis filter (AN69 ST) does not reduce clotting during hemodialysis when compared to aconventional polysulfone filter (F×8). Blood Purif. 2011;32:151–5.
Liu TM, Wu XZ, Qiu YR. Enhanced biocompatibility and antibacterial property of polyurethane materials modified withcitric acid and chitosan. J Biomater Sci Polym Ed. 2016;27:1211–31.
Major TC, Brisbois EJ, Jones AM, Zanetti ME, Annich GM, Bartlett RH, Handa H. The effect of a polyurethane coating incorporating both a thrombin inhibitor and nitric oxide onhemocompatibility in extracorporeal circulation. Biomaterials. 2014;35:7271–85.
Tang M, Xue J, Yan K, Xiang T, Zhao C. Heparin-like surface modification of polyethersulfone membrane and its biocompatibility. J Colloid Interface Sci. 2012;386:428–40.
Rozec B, Boissier E, Godier A, Cinotti R, Stephan F, Blanloeil Y. Argatroban, a new antithrombotic treatment for heparin-induced thrombocytopenia application in cardiac surgery and in intensive care. Ann Fr Anesth Reanim. 2014;33:514–23.
Siller-Matula JM, Schwameis M, Blann A, Mannhalter C, Jilma B. Thrombin as a multi-functional enzyme. Focus on in vitro and in vivo effects. Thrombosis & Haemostasis. 2011;105;1020–33.
Yixiong Z, Jianping N, Yanchao L, Siyuan D. Low dose of argatroban saline flushes anticoagulation in hemodialysis patients with high risk of bleeding. Clin Appl Thromb Hemost. 2010;16:440–5.
H. Toiserkani, G. Yilmaz, Y. Yagci, L. Torun. Functionalization of polysulfones by Click Chemistr446(2013)79–91m. Phys. 2010;211:2389–95.
Yue Wen-Wen, Li Hui-Juan, Xiang Tao, Qin Hui, Sun Shu-Dong, Zhao Chang-Sheng. Grafting of zwitterion from polysulfone membrane via surface-initiated ATRP with enhanced antifouling property and biocompatibility. J Membr Sci. 2013;446:79–91.
Xiang T, Zhang LS, Wang R, Xia Y, Su BH, Zhao CS. Blood compatibility comparison for polysulfone membranes modified by grafting block and random zwitterioniccopolymers via surface-initiated ATRP. J Colloid Interface Sci. 2014;432:47–56.
Kiessling V, Wan C, Tamm LK. Domain coupling in asymmetric lipid bilayers. Biochim Et Biophys Acta (BBA) - Biomembr. 2009;1788:64–71.
Rezaee R, Nasseri S, Mahvi AH, Nabizadeh R, Mousavi SA, Rashidi A, Jafari A, Nazmara S. Fabrication and characterization of a polysulfone-graphene oxide nanocomposite membrane for arsenate rejection from water. J Environ Health Sci & Eng. 2015;13:61.
Zhao ML, Li DJ, Guo MX, Zhang YT, Gu HQ. The different N concentrations induced cytocompatibility and hemocompatibility of MWCNTs with CNx coatings. Surf & Coat Technol. 2013;229:90–96.
Xin Tiana, Yun-Ren Qiu. 2-methoxyethylacrylate modified polysulfone membrane and its blood compatibility. Arch Biochem Biophys. 2017;631:49–57
Bain LE, Hoffmann MP, Bryan I, Collazo R, Ivanisevic A. Adsorption and adhesion of common serum proteins to nanotextured gallium nitride. Nanoscale . 2015;7:2360–5.
Wanderling C, Liles J, Finkler E, Carlsgaard P, Hopkinson W, Guler N, Hoppensteadt D, Fareed J. Dysregulation of tissue factor, thrombin-activatable fibrinolysis inhibitor, and fibrinogen in patients undergoing total joint arthroplasty. Clin Appl Thromb Hemost.2017;23:967–72.
Chen L, Yue J, Han X, Li J, Hu Y. Ouabain rescues rat nephrogenesis during intrauterine growth restriction by regulating the complement and coagulation cascades and calcium signaling pathway. J Dev Orig Health Dis. 2016;7:91–101.
Khan SU, Al-Saleh SS. Biochemical characterization of a factor X activator protein purified from Walterinnesia aegyptia venom. Blood Coagul Fibrinolysis. 2015;26:772–7.
Xiang T, Wang R, Zhao WF, Sun SD, Zhao CS. Covalent deposition of zwitterionic polymer and citric acid by click chemistry-enabled layer-by-layer assembly for improving the blood compatibility of polysulfone membrane. Langmuir. 2014;30:5115–25.
Adatya S, Sunny R, Fitzpatrick MJ, Colvin M, Thennapan T, John R, Dodge Zantek N, Pritzker M, Eckman P, Uriel N. Coagulation factor abnormalities related to discordance between anti-factor Xa and activated partial thromboplastin time in patients supported with continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2016;35:1311–20.
Wada H, Matsumoto T, Yamashita Y. Diagnosis of thrombosis by hemostatic markers. Nihon Rinsho. 2014;72:1232–6.
Sheriff J, Tran PL, Hutchinson M, DeCook T, Slepian MJ, Bluestein D, Jesty J. Repetitive Hypershear Activates and Sensitizes Platelets in a Dose-Dependent Manner. Artif Organs. 2015;40:586.
Austin KM, Covic L, Kuliopulos A. Matrix metalloproteases and PAR1 activation. Blood. 2013;121:431–9.
Hair PS, Sass LA, Vazifedan T, Shah TA, Krishna NK, Cunnion KM. Complement effectors, C5a and C3a, in cystic fibrosis lung fluid correlate with disease severity. PLoS One. 2017;12:e0173257.
GBT 16886-1997 Biological evaluation of medical devices Part 11: systemic toxicity test. 1997;3–7
Fey-Lamprecht F, Groth Th, Albrecht W, Paul D, Gross U. Development of membranes for the cultivation of kidney epithelial cells. Biomaterials. 2000;21:183–92.
Baumann H, Kokott A. Surface modification of the polymers present in a polysulfone hollow fiber hemodialyser by covalent binding of heparin or endothelial cell surface heparan sulfate: flow characteristics and platelet adhesion. J Biomater Sci Polym Edn. 2000;11:245–72.
Tardy-Poncet B, Nguyen P, Thiranos JC, Morange PE, Biron-Andréani C, Gruel Y, Morel J, Wynckel A, Grunebaum L, Villacorta-Torres J, de Maistre GS. Argatroban in the management of heparin-induced thrombocytopenia: a multicenter clinical trial. Crit Care. 2015;19:396.
Fu Q, Wu X, Kumar D, Ho JW, Kanhere PD, Srikanth N, Liu E, Wilson P, Chen Z. Development of sol-gel icephobic coatings: effect of surface roughness and surface energy. ACS Appl Mater Interfaces. 2014;6:20685–92.
Sun S, Yue Y, Huang. X, Meng D. Protein adsorption on blood-contact membrances. J Memb Sci. 2003;222:3–18.
Ostuni E, Chapman RG, Holmlin RE, Takayama S, Whitesides GM. A survey of structure–property relationshipsofsurfacesthatresisttheadsorptionof protein. Langmuir. 2001;17:5605–20.
DeAngelis RA, Reis ES, Ricklin D, et al. Targeted complement inhibition as a promising strategy for preventing inflammatory complications in hemodialysis. Immunobiology. 2012;217:1097–105.
Zhang Q, Lu X, Zhang Q, Zhang L, Li S. Liu S. Flux and Passage Enhancement in Hemodialysis by Incorporating Compound Additive into PVDF Polymer Matrix. Membr (Basel). 2016;19:6.
Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015;29:17–24.
Irfan M, Idris A. Overview of PES biocompatible/hemodialysis membranes: PES-blood interactions and modification techniques. Mater SciEng C Mater Biol Appl. 2015;56:574–92.
Xu XR, Zhang D, Oswald BE, Carrim N, Wang X, Hou Y, Zhang Q, Lavalle C, McKeown T, Marshall AH, Ni H. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit Rev Clin Lab Sci. 2016;53:409–30.
De Candia E. Mechanisms of platelet activation by thrombin: a short history. Thromb Res. 2012;129:250–6.
Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement system part I—molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, X., Ning, JP. Synthesis and biocompatibility of an argatroban-modified polysulfone membrane that directly inhibits thrombosis. J Mater Sci: Mater Med 29, 66 (2018). https://doi.org/10.1007/s10856-018-6054-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-018-6054-4