Abstract
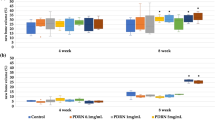
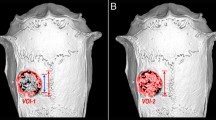
The osteoconduction of porous biphasic calcium phosphate (BCP) ceramics has been widely reported. In a previous study, we demonstrated that applying a nano-hydroxyapatite (nHA) coating enhances the osteoinductive potential of BCP ceramics, making these scaffolds more suitable for bone tissue engineering applications. The aim of the present study was to determine the effects of reconstructing radius defects in rabbits using nHA-coated BCP ceramics seeded with mesenchymal stem cells (MSCs) and to compare the bone regeneration induced by different scaffolds. Radius defects were created in 20 New Zealand rabbits, which were divided into four groups by treatment: porous BCP ceramics (Group A), nHA-coated porous BCP ceramics (Group B), porous BCP ceramics seeded with rabbit MSCs (Group C), and nHA-coated porous BCP ceramics seeded with rabbit MSCs (Group D). After in vitro incubation, the cell/scaffold complexes were implanted into the defects. Twelve weeks after implantation, the specimens were examined macroscopically and histologically. Both the nHA coating and seeding with MSCs enhanced the formation of new bone tissue in the BCP ceramics, though the osteoinductive potential of the scaffolds with MSCs was greater than that of the nHA-coated scaffolds. Notably, the combination of nHA coating and MSCs significantly improved the bone regeneration capability of the BCP ceramics. Thus, MSCs seeded into porous BCP ceramics coated with nHA may be an effective bone substitute to reconstruct bone defects in the clinic.










Similar content being viewed by others
References
Sen M, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury. 2007;38:S75–80.
Hartman EH, Spauwen PH, Jansen JA. Donor-site complications in vascularized bone flap surgery. J Invest Surg. 2002;15:185–97.
Rajan GP, Fornaro J, Trentz O, Zellweger R. Cancellous allograft versus autologous bone grafting for repair of comminuted distal radius fractures: a prospective, randomized trial. J Trauma. 2006;60:1322–9.
Hollinger JO, Schmitz JP, Mizgala JW, Hassler C. An evaluation of two configurations of tricalcium phosphate for treating craniotomies. J Biomed Mater Res. 1989;23:17–29.
Costantino PD, Friedman CD. Synthetic bone graft substitutes. Otolaryng Clin N Am. 1994;27:1037–74.
Daculsi G, Laboux O, Malard O, Weiss P. Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med. 2003;14:195–200.
Fellah BH, Weiss P, Gauthier O, Rouillon T, Pilet P, Daculsi G, et al. Bone repair using a new injectable self-crosslinkable bone substitute. J Orthop Res. 2006;24:628–35.
Werner J, Linner-Krčmar B, Friess W, Greil P. Mechanical properties and in vitro cell compatibility of hydroxyapatite ceramics with graded pore structure. Biomaterials. 2002;23:4285–94.
Marcacci M, Kon E, Zaffagnini S, Giardino R, Rocca M, Corsi A, et al. Reconstruction of extensive long-bone defects in sheep using porous hydroxyapatite sponges. Calcif Tissue Int. 1999;64:83–90.
Tampieri A, Celotti G, Sprio S, Delcogliano A, Franzese S. Porosity-graded hydroxyapatite ceramics to replace natural bone. Biomaterials. 2001;22:1365–70.
Habibovic P, Yuan H, van der Valk CM, Meijer G, van Blitterswijk CA, de Groot K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials. 2005;26:3565–75.
Yuan H, Kurashina K, de Bruijn JD, Li Y, De Groot K, Zhang X. A preliminary study on osteoinduction of two kinds of calcium phosphate ceramics. Biomaterials. 1999;20:1799–806.
Yuan H, Van Blitterswijk C, De Groot K, De Bruijn J. A comparison of bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) implanted in muscle and bone of dogs at different time periods. J Biomed Mater Res A. 2006;78:139–47.
Le Nihouannen D, Saffarzadeh A, Gauthier O, Moreau F, Pilet P, Spaethe R, et al. Bone tissue formation in sheep muscles induced by a biphasic calcium phosphate ceramic and fibrin glue composite. J Mater Sci Mater Med. 2008;19:667–75.
Habibovic P, Yuan H, van den Doel M, Sees TM, van Blitterswijk CA, de Groot K. Relevance of osteoinductive biomaterials in critical-sized orthotopic defect. J Orthop Res. 2006;24:867–76.
Habibovic P, Sees TM, van den Doel MA, van Blitterswijk CA, de Groot K. Osteoinduction by biomaterials—physicochemical and structural influences. J Biomed Mater Res A. 2006;77:747–62.
Habibovic P, Van der Valk C, Van Blitterswijk C, De Groot K, Meijer G. Influence of octacalcium phosphate coating on osteoinductive properties of biomaterials. J Mater Sci Mater Med. 2004;15:373–80.
Le Nihouannen D, Daculsi G, Saffarzadeh A, Gauthier O, Delplace S, Pilet P, et al. Ectopic bone formation by microporous calcium phosphate ceramic particles in sheep muscles. Bone. 2005;36:1086–93.
LeGeros R, Lin S, Rohanizadeh R, Mijares D, LeGeros J. Biphasic calcium phosphate bioceramics: preparation, properties and applications. J Mater Sci Mater Med. 2003;14:201–9.
Reddi AH. Morphogenesis and tissue engineering of bone and cartilage: inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000;6:351–9.
Ripamonti U. The morphogenesis of bone in replicas of porous hydroxyapatite obtained from conversion of calcium carbonate exoskeletons of coral. J Bone Joint Surg. 1991;73:692–703.
Kuboki Y, Takita H, Kobayashi D, Tsuruga E, Inoue M, Murata M, et al. BMP-Induced osteogenesis on the surface of hydroxyapatite with geometrically feasible and nonfeasible structures: topology of osteogenesis. J Biomed Mater Res. 1998;39:190–9.
Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21:1803–10.
Zandi M, Mirzadeh H, Mayer C, Urch H, Eslaminejad MB, Bagheri F, et al. Biocompatibility evaluation of nano-rod hydroxyapatite/gelatin coated with nano-HAp as a novel scaffold using mesenchymal stem cells. J Biomed Mater Res A. 2010;92A:1244–55.
Chen F, Lam W, Lin C, Qiu G, Wu Z, Luk K, et al. Biocompatibility of electrophoretical deposition of nanostructured hydroxyapatite coating on roughen titanium surface: in vitro evaluation using mesenchymal stem cells. J Biomed Mater Res B. 2007;82:183–91.
Guha AK, Singh S, Kumaresan R, Nayar S, Sinha A. Mesenchymal cell response to nanosized biphasic calcium phosphate composites. Colloid Surf B. 2009;73:146–51.
Hu J, Zhou Y, Huang L, Liu J, Lu H. Effect of nano-hydroxyapatite coating on the osteoinductivity of porous biphasic calcium phosphate ceramics. BMC Musculoskelet Disord. 2014;15:114.
Moreau JL, Xu HH. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate–chitosan composite scaffold. Biomaterials. 2009;30:2675–82.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7.
Dong J, Kojima H, Uemura T, Kikuchi M, Tateishi T, Tanaka J. In vivo evaluation of a novel porous hydroxyapatite to sustain osteogenesis of transplanted bone marrow-derived osteoblastic cells. J Biomed Mater Res. 2001;57:208–16.
Yoshikawa T, Ohgushi H, Tamai S. Immediate bone forming capability of prefabricated osteogenic hydroxyapatite. J Biomed Mater Res. 1996;32:481–92.
Ogura N, Kawada M, Chang W-J, Zhang Q, Lee S-Y, Kondoh T, et al. Differentiation of the human mesenchymal stem cells derived from bone marrow and enhancement of cell attachment by fibronectin. J Oral Sci. 2004;46:207–13.
Jenssen S, Broggini N, Weibrich G, Hjorting-Hanssen E, Shenk R, Buser D. Bone regeneration in standardized bone defects with autograft or bone substitute combination with platelet concentrate. A histologic and histomorphometeric study in the mandible of minipigs. Int J Oral Maxillofac Implants. 2005;20:703–12.
Vater C, Kasten P, Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7:463–77.
Kang S-H, Chung Y-G, Oh I-H, Kim Y-S, Min K-O, Chung J-Y. Bone regeneration potential of allogeneic or autogeneic mesenchymal stem cells loaded onto cancellous bone granules in a rabbit radial defect model. Cell Tissue Res. 2014;355:81–8.
The National Standard of the People’s Republic of China: Gb/T8489-2006: Test Method For Compressive Strength Of Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics). Beijing: China National Standardization Management Committee, 2006.
Ripamonti U. Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials. 1996;17:31–5.
Yuan H, Yang Z, Li Y, Zhang X, De Bruijn J, De Groot K. Osteoinduction by calcium phosphate biomaterials. J Mater Sci Mater Med. 1998;9:723–6.
Yuan H, Yang Z, de Bruijn JD, de Groot K, Zhang X. Material-dependent bone induction by calcium phosphate ceramics: a 2.5-year study in dog. Biomaterials. 2001;22:2617–23.
Hanawa T, Kamiura Y, Yamamoto S, Kohgo T, Amemiya A, Ukai H, et al. Early bone formation around calcium-ion-implanted titanium inserted into rat tibia. J Biomed Mater Res. 1997;36:131–6.
Zomorodian E, Baghaban Eslaminejad M. Mesenchymal stem cells as a potent cell source for bone regeneration. Stem Cells Int. 2012;2012:980353.
Schopper C, Ziya-Ghazvini F, Goriwoda W, Moser D, Wanschitz F, Spassova E, et al. HA/TCP compounding of a porous CaP biomaterial improves bone formation and scaffold degradation—A long-term histological study. J Biomed Mater Res B. 2005;74:458–67.
Arinzeh TL, Peter SJ, Archambault MP, Van Den Bos C, Gordon S, Kraus K, et al. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg. 2003;85:1927–35.
Gamblin A-L, Brennan MA, Renaud A, Yagita H, Lézot F, Heymann D, et al. Bone tissue formation with human mesenchymal stem cells and biphasic calcium phosphate ceramics: the local implication of osteoclasts and macrophages. Biomaterials. 2014;35:9660–7.
Shi Z, Huang X, Cai Y, Tang R, Yang D. Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater. 2009;5:338–45.
Cai Y, Liu Y, Yan W, Hu Q, Tao J, Zhang M, et al. Role of hydroxyapatite nanoparticle size in bone cell proliferation. J Mater Chem. 2007;17:3780–7.
Lee HJ, Choi HW, Kim KJ, Lee SC. Modification of hydroxyapatite nanosurfaces for enhanced colloidal stability and improved interfacial adhesion in nanocomposites. Chem Mater. 2006;18:5111–8.
Zavgorodniy AV, Borrero-López O, Hoffman M, LeGeros RZ, Rohanizadeh R. Characterization of the chemically deposited hydroxyapatite coating on a titanium substrate. J Mater Sci Mater Med. 2011;22:1–9.
Wang D-X, He Y, Bi L, Qu Z-H, Zou J-W, Pan Z, et al. Enhancing the bioactivity of Poly (lactic-co-glycolic acid) scaffold with a nano-hydroxyapatite coating for the treatment of segmental bone defect in a rabbit model. Int J Nanomed. 2013;8:1855.
Li B, Chen X, Guo B, Wang X, Fan H, Zhang X. Fabrication and cellular biocompatibility of porous carbonated biphasic calcium phosphate ceramics with a nanostructure. Acta Biomater. 2009;5:134–43.
Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices. 2006;3(1):49–57.
Viateau V, Guillemin G, Bousson V, Oudina K, Hannouche D, Sedel L, et al. Long-bone critical-size defects treated with tissue-engineered grafts: a study on sheep. J Orthop Res. 2007;25:741–9.
Acknowledgments
This study was funded by The National High Technology Research and Development Program of China (2013AA032203), Science and technology Commission of Hunan Province of China (2013GK2002), China Changsha Science and Technology Projects (K1101025-31), and the open projects of State Key Laboratory of Powder Metallurgy, Central South University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, J., Yang, Z., Zhou, Y. et al. Porous biphasic calcium phosphate ceramics coated with nano-hydroxyapatite and seeded with mesenchymal stem cells for reconstruction of radius segmental defects in rabbits. J Mater Sci: Mater Med 26, 257 (2015). https://doi.org/10.1007/s10856-015-5590-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5590-4