Abstract
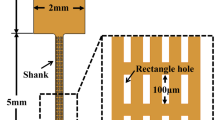
In vivo insertion experiments are essential to optimize novel neural implants. Our work focuses on the interaction between intact dura mater of rats and as-fabricated single-shaft silicon microprobes realized by deep reactive ion etching. Implantation parameters like penetration force and dimpling through intact dura mater were studied as a function of insertion speed, microprobe cross-section, tip angle and animal age. To reduce tissue resistance, we proposed a unique tip sharpening technique, which was also evaluated in in vivo insertion tests. By doubling the insertion speed (between 1.2 and 10.5 mm/min), an increase of 10–35 % in penetration forces was measured. When decreasing the cross-section of the microprobes, penetration forces and dimpling was reduced by as much as 30–50 % at constant insertion speeds. Force was noticed to gradually decrease by decreasing tip angles. Measured penetration forces through dura mater were reduced even down to 11 ± 3 mN compared to unsharpened (49 ± 13 mN) probes by utilizing our unique tip sharpening technique, which is very close to exerted penetration force in the case of retracted dura (5 ± 1.5 mN). Our findings imply that age remarkably alters the elasticity of intact dura mater. The decreasing stiffness of dura mater results in a significant rise in penetration force and decrease in dimpling. Our work is the first in vivo comparative study on microelectrode penetration through intact and retracted dura mater.







Similar content being viewed by others
References
Abolhassani N, Patel R, Moallema M. Needle insertion into soft tissue: a survey. Med Eng Phys. 2007;29:413–31.
Aggarwal P, Johnston CR. Geometrical effects in mechanical characterizing of microneedle for biomedical applications. Sens Actuator B. 2004;102:226–34.
Andrei A, Welkenhuysen M, Nuttin B, Eberle W. A response surface model predicting in vivo insertion behaviour of micromachined neural implants. J Neural Eng. 2012;9:016005.
Bai Q, Wise KD, Anderson DJ. A high-yield microassembly structure for three-dimensional microelectrode arrays. IEEE Trans Biomed Eng. 2000;47:281–9.
Bjornsson CS, Oh SJ, Al-Kofahi YA, Lim YJ, Smith KL, Turner JN, De S, Roysam B, Shain W, Kim SJ. Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. J Neural Eng. 2006;3:196–207.
Cham JG, Branchaud EA, Nenadic Z, Greger B, Andersen RA, Burdick JW. Semi-chronic motorized microdrive and control algorithm for autonomously isolating and maintaining optimal extracellular action potentials. J Neurophysiol. 2004;93:570–9.
Cheung KC, Renaud P, Tanila H, Djupsund K. Flexible polyimide microelectrode array for in vivo recordings and current source density analysis. Biosens Bioelectron. 2007;22:1783–90.
Davis SP, Landisa BJ, Adams ZH, Allen MG, Prausnitz MR. Insertion of microneedles into skin: measurement and prediction of insertion force and needle fracture force. J Biomech. 2004;37:1155–63.
Edell DJ, Toi VV, McNeil VM, Clark LD. Factors influencing the biocompatibility of insertable silicon microshafts in cerebral cortex. IEEE Trans Biomed Eng. 1992;39:635–43.
Fee MS, Leonardo A. Miniature motorized microdrive and commutator system for chronic neural recording in small animals. J Neurosci Method. 2001;112:83–94.
Fekete Z, Hajnal Z, Marton G, Fürjes P, Pongracz A. Fracture analysis of silicon microprobes designed for deep-brain stimulation, Microelectron Eng. 2013;103:160–6.
Gefen A, Margulies SS. Are in vivo and in situ brain tissues mechanically similar? J Biomech. 2004;37:1339–52.
Gefen A, Gefen N, Zhu Q, Raghupathi R, Margulies SS. Age-dependent changes in material properties of the brain and braincase of the rat. J Neurotrauma. 2003;11:1163–77.
Grand L, Pongrácz A, Vázsonyi É, Márton G, Gubán D, Fiáth R, Kerekes BP, Karmos G, Ulbert I, Battistig G. A novel multisite silicon probe for high quality laminar neural recordings. Sens Actuator A. 2011;166:14–21.
Hajjhassan M, Chodavarapu V, Musallam S. NeuroMEMS: neural probe microtechnologies. Sensors. 2008;8:6704–26.
Henderson JH, Nacamuli RP, Zhao B, Longaker MT, Carter DR. Age-dependent residual tensile strains are present in the dura mater of rats. J R Soc Interface. 2005;2:159–67.
Hosseini NH, Hoffmann R, Kisban S, Stieglitz T, Paul O, Ruther P. Comparative study on the insertion behaviour of cerebral microprobes. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:4711–4.
Jansen HV, de Boer MJ, Wiegerink R, Tas N, Smulders E, Neagu C, Elwenspoek M. RIE lag in high aspect ratio trench etching of silicon. Microelectron Eng. 1997;35:45–50.
Jensen W, Yoshida K, Hofmann UG. In-vivo implant mechanics of flexible, silicon-based ACREO microelectrode arrays in rat cerebral cortex. IEEE Trans Biomed Eng. 2006;53:934–40.
Johnson MD, Kao OE, Kipke DR. Spatio temporal pH dynamics following insertion of neural microelectrode arrays. J Neurosci Methods. 2007;160:276–87.
Misra S, Reed KB, Douglas AS, Ramesh KT, Okamura AM. Needle-tissue interaction forces for bevel-tip steerable needles. Proc IEEE RAS EMBS Int Conf Biomed Robot Biomechatron, Scottsdale, USA; 2008. p. 224–31.
Najafi K. Strength characterization of silicon microprobes in neurophysiological tissues. IEEE Trans Biomed Eng. 1990;37:474–81.
Paralikar KJ, Clement RS. Collagenase-aided intracortical microelectrode array insertion: effects on insertion force and recording performance. IEEE Trans Biomed Eng. 2008;55:2258–67.
Pongrácz A, Fekete Z, Márton G, Bérces Z, Ulbert I, Fürjes P. Deep-brain silicon multielectrodes for simultaneous in vivo neural recordingand drug delivery. Sensor Actuat B. 2013;189:97–105
Rennaker RL, Street S, Ruyle AM, Sloan AM. A comparison of chronic multi-channel cortical implantation techniques: manual versus mechanical insertion. J Neurosci Methods. 2005;142:169–76.
Rousche PJ, Normann RA. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Annals Biomed Eng. 1992;20:413–22.
Scott KM, Du J, Lester HA, Masmanidis SC. Variability of acute extracellular action potential measurements with multisite. J Neurosci Methods. 2012;211:22–30.
Seidl K, Herwik S, Torfs T, Neves HP, Paul O, Ruther P. CMOS-based high-density silicon microprobe arrays for electronic depth control in intracortical neural recording. J Microelectromech Syst. 2011;20:1439–48.
Sharp AA, Ortega AM, Restrepo D, Curran-Everett D, Gall K. In vivo penetration mechanics and mechanical properties of mouse brain tissue at micrometer scales. Trans Biomed Eng. 2009;56:45–53.
Spieth S, Brett O, Seidl K, Aarts AAA, Erismis MA, Herwik S, Trenkle F, Tatzner S, Auber J, Daub M, Neves HP, Puers R, Paul O, Ruther P, Zengerle R. A floating 3D silicon microprobe array for neural drug delivery compatible with electrical recording. J Micromech Microeng. 2011;21:125001.
Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN, Shain W. Brain responses to micro-machined silicon devices. Brain Res. 2003;983:23–35.
Tijero M, Gabriel G, Caro J, Altuna A, Hernández R, Villa R, Berganzo J, Blanco FJ, Salido R, Fernández LJ. SU-8 microprobe with microelectrodes for monitoring electrical impedance in living tissues. Biosens Bioelectron. 2009;24:2410–6.
Van Noort R, Martin TRP, Black MM, Barker AT, Montero CG. The mechanical properties of human dura mater and the effects of storage media. Clin Phys Physiol Meas. 1981;2:197.
Vázsonyi É, Vértesy Z, Tóth A, Szlufcik J. Anisotropic etching of silicon in a two-component alkaline solution. J Micromech Microeng. 2003;13:165–9.
Vázsonyi É, C Dücső, Pekker A. Characterization of the anisotropic etching of silicon in two-component alkaline solution. J Micromech Microeng. 2007;17:1916.
Welkenhuysen M, Andrei AA, Ameye L, Eberle W, Nuttin B. Effect of insertion speed on tissue response and insertion mechanics of a chronically implanted silicon-based neural probe. IEEE Trans Biomed Eng. 2011;58:3250–9.
Acknowledgments
The authors are grateful to the supportive staff of MEMS Lab. A. Pongrácz is thankful for the Bolyai János Grant of the HAS. Z. Fekete is supported by the Postdoctoral Research Programme of the HAS and the Alexander von Humboldt Foundation. The funding of the Hungarian Science Foundation (OTKA K81354) to I. Ulbert and the Hungarian Brain Research Program (KTIA NAP) are also highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fekete, Z., Németh, A., Márton, G. et al. Experimental study on the mechanical interaction between silicon neural microprobes and rat dura mater during insertion. J Mater Sci: Mater Med 26, 70 (2015). https://doi.org/10.1007/s10856-015-5401-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5401-y