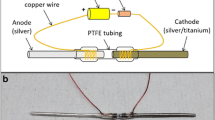
Abstract
Post-operative infection is a major risk associated with implantable devices. Prior studies have demonstrated the effectiveness of ionic silver as an alternative to antibiotic-based infection prophylaxis and treatment. The focus of this study is on an electrically activated implant system engineered for active release of antimicrobial silver ions. The objective was to evaluate the effects of the cathode design, especially the cathode material, on the in vitro antimicrobial efficacy of the system. A modified Kirby-Bauer diffusion technique was used for the antimicrobial efficacy evaluations (24 h testing interval). In phase-1 of the study, a three-way ANOVA (n = 6, α = 0.05) was performed to determine the effects of cathode material (silver, titanium, and stainless steel), cathode surface area and electrode separation distance on the efficacy of the system against Staphylococcus aureus. The results show that within the design space tested, none of these parameters had a statistically significant effect on the antimicrobiality of the system (P > 0.15). Subsequently, one-way ANOVA (n = 6, α = 0.05) was conducted in phase-2 to validate the inference regarding the non-significance of the cathode material to the system efficacy using a broader spectrum of pathogens (methicillin-resistant S. aureus, Escherichia coli, Streptococcus agalactiae and Aspergillus flavus) responsible for osteomyelitis. The results confirmed the lack of statistical difference between efficacies of the three cathode material configurations against all pathogens tested (P > 0.58). Overall, the results demonstrate the ability to alter the cathode material and related design parameters in order to minimize the silver usage in the system without adversely affecting its antimicrobial efficacy.







Similar content being viewed by others
References
Center for Disease Control—National Center for Health Statistics. 2013. http://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf. Accessed 9 Aug 2013.
Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Jt Surg Am. 2007;89:780–5.
The Fredonia Group. Implantable medical devices: industry study with forecasts for 2015 and 2020. Cleveland: Fredonia; 2012.
Ehrlich GD, Stewart PS, Post JC, Lin Q, Stoodley P, Kathju S, Zhao Y, McLeod BR, Balaban N, Hu FZ, Sotereanos NG, Costerton JW. Engineering approaches for the detection and control of orthopaedic biofilm infections. Clin Orthop Relat Res. 2005;437:59–66.
Shirwaiker RA, Samberg ME, Cohen PH, Wysk RA, Monteiro-Riviere NA. Nanomaterials and synergistic low-intensity direct current (LIDC) stimulation technology for orthopedic implantable medical devices. Wiley Interdiscip Rev. 2013;5:191–204.
Trampuz A, Zimmerli W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury. 2006;37:S59–66.
Lavernia C, Lee DJ, Hernandez VH. The increasing financial burden of knee revision surgery in the United States. Clin Orthop Relat Res. 2006;446:221–6.
Cavanaugh DL, Berry J, Yarboro SR, Dahners LE. Better prophylaxis against surgical site infection with local as well as systemic antibiotics. J Bone Jt Surg Am. 2009;91:1907–12.
Kumar R, Munstedt H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials. 2005;26:2081–8.
Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35:780–9.
Monteiro DR, Gorup LF, Takamiya AS, Ruvollo AC, Camargo ER, Barbosa DB. The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. Int J Antimicrob Agents. 2009;34:103–10.
Chopra I. Controlled release of biologically active silver from nanosilver surfaces. J Antimicrob Chemother. 2010;4:6903–13.
Spadaro JA, Berger TJ, Barranco SD, Chapin SE, Becker RO. Antibacterial effects of silver electrodes with weak direct current. Antimicrob Agents Chemother. 1974;6:637–42.
Becker RO, Spadaro JA. Treatment of orthopaedic infections with electrically generated silver ions. J Bone Jt Surg Am. 1978;60:871.
Raad I, Hachem R, Zermeno A, Stephens LC, Bodey GP. Silver iontophoretic catheter: a prototype of a long-term anti-infective vascular access device. J Infect Dis. 1996;173:495–8.
Oyama T, Nakano MH, Arai T, Kato D, Maeda N. In vitro evaluation of antimicrobial efficacy of iontophoresis against Enterococcus faecalis, Candida albicans, Pseudomonas aeruginosa and Bacillus subtilis. J Oral Biosci. 2009;5:91–6.
Fuller TA, Wysk RA, Charumani C, Kennett M, Sebastiennelli WJ, Abrahams R, Shirwaiker RA, Voigt RC, Royer P. Developing an engineered antimicrobial/prophylactic system using electrically activated bactericidal metals. J Mater Sci Mater Med. 2010;21:2103–14.
Shirwaiker RA, Wysk RA, Kariyawasam S, Carrion H, Voigt RC. Micro-scale fabrication and characterization of a silver-polymer-based electrically activated antibacterial surface. Biofabrication. 2011;3:015003.
Wysk RA, Sebastianelli WJ, Shirwaiker RA, Bailey GM, Charumani C, Kennett M, Kaucher A, Abrahams R, Fuller TA, Royer P, Voigt RC, Cohen PH. Prophylactic bactericidal orthopedic implants—animal testing study. J Biomed Sci Eng. 2010;3:917–26.
Milder FL, Anderson D, Weitzner BD. Iontophoretic material. June 1998. US patent 5759564 A.
Berger TJ, Spadaro JA, Chapin SE, Becker RO. Electrically generated silver ions: quantitative effects on bacterial and mammalian cells. Antimicrob Agents Chemother. 1976;9:357–8.
Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001;2:S96–101.
Hardes J, Winkelmann W, Gosheger G, Ahrens H, Gebert C, Streitbuerger A, Buerger H, Erren M, Gunsel A, Wedemeyer C, Saxler G. Lack of toxicological side-effects in silver-coated megaprostheses in humans. Biomaterials. 2007;28:2869–75.
Samberg ME, Tan Z, Monteiro-Riviere NA, Orndorff PE, Shirwaiker RA. Biocompatibility analysis of an electrically-activated silver-based antibacterial surface system for medical device applications. J Mater Sci Mater Med. 2013;24:755–60.
Hayes JS, Richards RG. The use of titanium and stainless steel in fracture fixation. Expert Rev Med Devices. 2010;7:843–53.
Wall EJ, Jain V, Vora V, Mehlman CT, Crawford AH. Complications of titanium and stainless steel elastic nail fixation of pediatric femoral fractures. J Bone Jt Surg Am. 2008;90:1305–13.
Oh K, Kim Y, Park Y, Kim K. Properties of super stainless steels for orthodontic applications. J Biomed Mater Res B. 2004;69:183–94.
Pieske O, Geleng P, Zaspel J, Piltz S. Titanium alloy pins versus stainless steel pins in external fixation at the wrist: a randomized prospective study. J Trauma. 2008;64:1275–80.
Bertrand X, Slekovec C, Talon D. Use of mupirocin-chlorhexidine treatment to prevent Staphylococcus aureus surgical-site infections. Fut Microbiol. 2010;5:701–3.
Keppel G, Wickens TD. Design and analysis: a researcher’s handbook (4th edition). London: Pearson; 2004.
Donlan R. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7:277–81.
Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol. 2012;65:158–68.
John A, Baldoni D, Haschke M, Rentsch K, Schaerli P, Zimmerli W, Trampuz A. Efficacy of daptomycin in implant-associated infection due to methicillin-resistant Staphylococcus aureus: importance of combination with rifampin. Antimicrob Agents Chemother. 2009;53:2719–24.
Engemann JJ, Carmeli Y, Cosgrove SE, Fowler VG, Bronstein MZ, Trivette SL, Briggs JP, Sexton DJ, Kaye KS. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis. 2003;36:592–8.
Liu C, Murray BE, Rybak M, Talan DA, Chambers HF, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–55.
Pasqualotto AC, Denning DW. Post-operative aspergillosis. Clin Microbiol Infect. 2006;12:1060–76.
Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology. 2007;153:1677–92.
DeVries JG, Cuttica DJ, Hyer CF. Cannulated screw fixation of Jones fifth metatarsal fractures: a comparison of titanium and stainless steel screw fixation. J Foot Ankle Surg. 2011;50:207–12.
Cieślik M, Engvall K, Pan J, Kotarba A. Silane–parylene coating for improving corrosion resistance of stainless steel 316L implant material. Corros Sci. 2011;53:296–301.
Long M, Rack HJ. Titanium alloys in total joint replacement—a materials science perspective. Biomaterials. 1998;19:1621–39.
Bagchi D, Bagchi M, Stohs SJ. Chromium (VI)-induced oxidative stress, apoptotic cell death and modulation of p53 tumor suppressor gene. Mol Cell Biochem. 2001;222:149–58.
Niinomi M. Recent metallic materials for biomedical applications. Metall Mater Trans A. 2002;33:477–86.
Carlsson L, Röstlund T, Albrektsson B, Albrektsson T, Brånemark PI. Osseointegration of titanium implants. Acta Orthop Scand. 1986;57:285.
Yang Y, Bumgardner J, Haggard W, Ong J, Oh N, Liu Y, Chen W, Oh S, Appleford M, Kim S, Kim K, Park S. Enhancing osseointegration using surface-modified titanium implants. JOM. 2006;58:71–6.
Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23:844–54.
Masri BA, Duncan CP, Beauchamp CP. Long-term elution of antibiotics from bone-cement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplast. 1998;13:331–8.
Lombardi AV, Berend KR, Adams JB, Karnes JM. Articulating antibiotic spacers: the standard of care for an infected total knee arthroplasty. Orthopedics. 2007;30:786–7.
Kim DK, Kim IS, Kim SJ, Song YM, Song JK, Zhang YL, Lee TH, Cho TH, Hwang SJ. Biphasic electric current stimulates proliferation and induces VEGF production in osteoblasts. BBA Mol Cell Res. 2006;1769:907–16.
Song JK, Cho TH, Pan H, Song YM, Kim IS, Lee TH, Hwang SJ, Kim SJ. An electronic device for accelerating bone formation in tissues surrounding a dental implant. Bioelectromagnetics. 2009;30:374–84.
Acknowledgments
This work was supported by a research Grant from NC State’s 2013 Research and Innovation and Seed Funding (RISF) program. The authors thank Ms. Patty Spears and Ms. Mitsu Suyemoto from NC State University’s College of Veterinary Medicine, and Dr. Gary Payne, Mr. Gregory O’Brian and Dr. Xiaomei Shu from NC State University’s Department of Plant Pathology for their valuable and constructive suggestions during the antimicrobial efficacy testing experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, Z., Ganapathy, A., Orndorff, P.E. et al. Effects of cathode design parameters on in vitro antimicrobial efficacy of electrically-activated silver-based iontophoretic system. J Mater Sci: Mater Med 26, 44 (2015). https://doi.org/10.1007/s10856-015-5382-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5382-x