Abstract
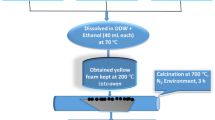
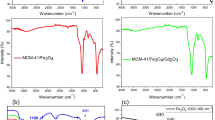
Hyperthermia is one of the most recents therapies for cancer treatment using particles with nanometric size and appropriate magnetic properties for destroying cancer cells. Magnetic nanoparticles (MNP’s) of Fe–Ga and synthesized using a polycondensation reaction by sol–gel method were obtained. MNP’s of Fe1.4Ga1.6O4 that posses an inverse spinel structure were identified by X-Ray Diffraction, Transmission Electron Microscopy, Scanning Electron Microscopy and Energy Dispersive Spectroscopy. The results showed that the MNP’s are composed only by Fe, Ga and O and their size is between 15 and 20 nm. The magnetic properties measured by Vibration Sample Magnetometry demonstrated a saturation magnetization value of 37.5 emu/g. To induce the MNP’s bioactivity, a biomimetic method was used which consisted in the immersion of MNP’s in a Simulated Body Fluid (SBF) for different periods of time (7, 14 and 21d) along with a wollastonite disk. The formation of a bioactive layer, which closely resembles that formed on the existing bioactive systems and with a Ca/P atomic ratio within a range of 1.37–1.73 was observed on the MNP’s. Cytotoxicity of MNP’s was evaluated by in vitro hemolysis testing using human red blood cells at concentrations between 0.25 and 6.0 mg/mL. It was found that the MNP’s were not cytotoxic at none of the concentrations used. The results indicate that Fe–Ga MNP’s are potential materials for cancer treatment of both hard and soft tissue by hyperthermia and drug carriers, among other applications.




Similar content being viewed by others
References
Jemal A, Siegel R. Cancer Facts and Figures 2011. Atlanta: American Cancer Society, Inc.; 2011.
Macarulla MT, Javier PF. Comprender el cancer. Editorial AMAT; 2009.
Chicheł A, Skowronek J, Kubaszewska M, Kanikowski M. Hyperthermia—description of a method and a review of clinical applications. Rep Pract Oncol Radiother. 2007;12(5):267–75. doi:10.1016/S1507-1367(10)60065-X..
Hernández AV, Quero JEC, Salas LL, Mier YH, Marchal C, Eléctrica SB. Hipertermia electromagnética, una alternativa para el tratamiento del cáncer: antecedentes, aspectos físicos y biológicos. Rev Mex Ing Biom. 2001;002:78–88.
Medeiros S, Santos A, Fessi H, Elaissari A. Stimuli-responsive magnetic particles for biomedical applications. Int J Pharm. 2011;403(1):139–61.
Faiyas A, Vinod E, Joseph J, Ganesan R, Pandey R. Dependence of pH and surfactant effect in the synthesis of magnetite (Fe3O4) nanoparticles and its properties. J Magn Magn Mater. 2010;322(4):400–4.
Fraga AF, Bini RA, Guastaldi AC. Bioactive coating on titanium implants modified by Nd:YVO4 laser. Appl Surf Sci. 2011;257(10):4575–80.
Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev. 2011;63(1):24–46.
Figuerola A, Di Corato R, Manna L, Pellegrino T. From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharm Res. 2010;62(2):126–43.
Sun S, Zeng H. Size-controlled synthesis of magnetite nanoparticles. J Am Chem Soc. 2002;124(28):8204–5.
Shinkai M, Yanase M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: in vitro study. Cancer Sci. 1996;87(11):1179–83.
Rosen JE, Chan L, Shieh D-B, Gu FX. Iron oxide nanoparticles for targeted cancer imaging and diagnostics. Nanomed Nanotechnol Biol Med. 2012;8(3):275–90.
Ma M, Zhang Y, Yu W, Shen H-y, Zhang H-q, Gu N. Preparation and characterization of magnetite nanoparticles coated by amino silane. Coll Surf A. 2003;212(2):219–26.
Singh RK, Kim TH, Patel KD, Knowles JC, Kim HW. Biocompatible magnetite nanoparticles with varying silica-coating layer for use in biomedicine: physicochemical and magnetic properties, and cellular compatibility. J Biomed Mater Res A. 2012;100(7):1734–42.
Baba D, Seiko Y, Nakanishi T, Zhang H, Arakaki A, Matsunaga T, et al. Effect of magnetite nanoparticles on living rate of MCF-7 human breast cancer cells. Coll Surf B. 2012;95:254–7.
Kawashita M, Tanaka M, Kokubo T, Inoue Y, Yao T, Hamada S, et al. Preparation of ferrimagnetic magnetite microspheres for in situ hyperthermic treatment of cancer. Biomaterials. 2005;26(15):2231–8.
Escobedo-Bocardo JC, Cortés-Hernández DA, Múzquiz-Ramos E, Herrera-Romero O, editors. Preparation and properties of CoFe2O4 synthesized by the modified citrate-gel method. Materials Science Forum; 2010: Trans Tech Publ.
Stoia M, Barvinschi P, Tudoran LB, Barbu M, Stefanescu M. Synthesis of nanocrystalline nickel ferrite by thermal decomposition of organic precursors. J Therm Anal Calorim. 2012;108(3):1033–9.
Sheykhan M, Mohammadnejad H, Akbari J, Heydari A. Superparamagnetic magnesium ferrite nanoparticles: a magnetically reusable and clean heterogeneous catalyst. Tetrah Lett. 2012;53(24):2959–64.
Huang C-C, Su C-H, Liao M-Y, Yeh C-S. Magneto-optical FeGa2O4 nanoparticles as dual-modality high contrast efficacy T2 imaging and cathodoluminescent agents. Phys Chem Chem Phys. 2009;11(30):6331–4.
Yang H, Zhang C, Shi X, Hu H, Du X, Fang Y, et al. Water-soluble superparamagnetic manganese ferrite nanoparticles for magnetic resonance imaging. Biomaterials. 2010;31(13):3667–73.
Warrell RP. Gallium nitrate for the treatment of bone metastases. Cancer. 1997;80(S8):1680–5.
Gómez-Ruiz S, Gallego B, Kaluđerović MR, Kommera H, Hey-Hawkins E, Paschke R, et al. Novel gallium (III) complexes containing phthaloyl derivatives of neutral aminoacids with apoptotic activity in cancer cells. J Organomet Chem. 2009;694(14):2191–7.
Enyedy ÉA, Dömötör O, Varga E, Kiss T, Trondl R, Hartinger CG, et al. Comparative solution equilibrium studies of anticancer gallium (III) complexes of 8-hydroxyquinoline and hydroxy (thio) pyrone ligands. J Inorg Biochem. 2012;117:189–97.
Jakupec MA, Galanski M, Arion VB, Hartinger CG, Keppler BK. Antitumour metal compounds: more than theme and variations. Dalton Trans. 2008;2:183–94.
Múzquiz-Ramos EM, Cortés-Hernández D, Escobedo-Bocardo J, Zugasti-Cruz A, Ramírez-Gómez X, Osuna-Alarcón J. In vitro and in vivo biocompatibility of apatite-coated magnetite nanoparticles for cancer therapy. J Mater Sci Mater Med. 2013;24(4):1035–41.
Muzquiz-Ramos E, Cortes-Hernandez D, Escobedo-Bocardo J, Zugasti-Cruz A. In vitro bonelike apatite formation on magnetite nanoparticles after a calcium silicate treatment: preparation, characterization and hemolysis studies. Ceram Int. 2012;38(8):6849–56.
Martinez A, Izquierdo-Barba I, Vallet-Regi M. Bioactivity of a CaO-SiO2 binary glasses system. Chem Mater. 2000;12(10):3080–8.
F756-00 A. Standard practice for assessment of hemolytic properties of materials. West Conshohocken: ASTM International West Conshohocken; 2000.
Sánchez HJ. Síntesis de materiales magnéticos por sol–gel a partir de precursores de Fe y Ga para aplicaciones en hipertermia CINVESTAV Unidad-Saltillo; 2012.
Múzquiz-Ramos E, Cortés-Hernández DA, Sánchez-Torres C, Escobedo-Bocardo JC, Zugasti A, Ramírez-Gómez X. Biomimetic magnetic nanoparticles for hyperthermia treatment. Key Eng Mater. 2012;493:16–9.
Brusentsov NA, Gogosov V, Brusentsova T, Sergeev A, Jurchenko N, Kuznetsov AA, et al. Evaluation of ferromagnetic fluids and suspensions for the site-specific radiofrequency-induced hyperthermia of MX11 sarcoma cells in vitro. J Magn Magn Mater. 2001;225(1):113–7.
Gkanas EI. In vitro magnetic hyperthermia response of iron oxide MNP’s incorporated in DA3, MCF-7 and HeLa cancer cell lines. Cent Eur J Chem. 2013;11(7):1042–54.
Ginebra Molins MP. Desarrollo y caracterización de un cemento óseo basado en fosfato tricácico para aplicaciones quirúrgicas. Universitat Politècnica de Catalunya; 1997.
Múzquiz-Ramos EM, Cortés-Hernández DA, Escobedo-Bocardo J. Biomimetic apatite coating on magnetite particles. Mater Lett. 2010;64(9):1117–9.
Acknowledgments
The authors gratefully acknowledge CONACYT, México for the provision of the Héctor Javier Sánchez Fuentes scholarship and SEP-CONACYT (Basic Science project, 127815) and Goval Industrial, S.A. de C.V. for the financial support for this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sánchez, J., Cortés-Hernández, D.A., Escobedo-Bocardo, J.C. et al. Bioactive magnetic nanoparticles of Fe–Ga synthesized by sol–gel for their potential use in hyperthermia treatment. J Mater Sci: Mater Med 25, 2237–2242 (2014). https://doi.org/10.1007/s10856-014-5197-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-014-5197-1