Abstract
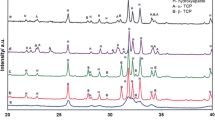
α-Tricalcium phosphate (α-TCP) has become the main reactant of most experimental and commercial ceramic bone cements. It has calcium-to-phosphorus (Ca/P) ratio of 1.50. The present study expands and reports on the microstructures and mechanical properties of calcium phosphate (CP) cements containing sintered monolithic reactants obtained in the interval 1.29 < Ca/P < 1.77. The study focuses on their cement setting and hardening properties as well as on their microstructure and crystal phase evolution. The results showed that: (a) CP-cements made with reactants with Ca/P ratio other than 1.50 have longer setting and lower hardening properties; (b) CP-cements reactivity was clearly affected by the Ca/P ratio of the starting reactant; (c) reactants with Ca/P < 1.50 were composed of several phases, calcium pyrophosphate and α- and β-TCP. Similarly, reactants with Ca/P > 1.50 were composed of α-TCP, tetracalcium phosphate and hydroxyapatite; (d) only the reactant with Ca/P = 1.50 was monophasic and was made of α-TCP, which transformed during the setting into calcium deficient hydroxyapatite; (e) CP-cements developed different crystal microstructures with specific features depending on the Ca/P ratio of the starting reactant.









Similar content being viewed by others
References
Vallet-Regí M, González-Calbet JM. Calcium phosphates as substitution for bone tissues. Prog Solid State Chem. 2004;32:1–31. doi:10.1016/j.progsolidstchem.2004.07.001.
Vallet-Regí M. Ceramic for medical applications. J Chem Soc. 2001;17:97–108. doi:10.1039/B007852M.
Fernández E. Bioactive bone cements. In: Akay M, editor. Wiley encyclopedia of biomedical engineering, ISBN: 978-0-471-24967-2, vol. 1. New York: Wiley; 2006. p. 345–54. doi:10.1002/9780471740360.ebs1367.
De Aza PN, Guitián F, De Aza S. Bioeutectic: a new ceramic material for human bone replacement. Biomaterials. 1997;18:1285–91. doi:10.1016/S0142-9612(97)00063-X.
Alemany MI, Velasquez P, de la Casa-Lillo MA, De Aza PN. Effect of materials’ processing methods on the ‘in vitro’ bioactivity of wollastonite glass-ceramic materials. J Non Cryst Solids. 2005;351:1716–26. doi:10.1016/j.jnoncrysol.2005.04.062.
Dorozhkin SV. A review on the dissolution models of calcium apatites. Prog Cryst Growth Charact Mater. 2002;44(1):45–61. doi:10.1016/S0960-8974(02)00004-9.
Petrov OE, Dyulgerova E, Petrov L, Popova R. Characterization of calcium phosphate phases obtained during the preparation of sintered biphase Ca–P ceramics. Mater Lett. 2001;48:162–7. doi:10.1016/S0167-577X(00)00297-4.
Dorozhkina EI, Dorozhkin SV. Mechanism of the solid-state transformation of a calcium-deficient hydroxyapatite (CDHA) into biphasic calcium phosphate (BCP) at elevated temperatures. Chem Mater. 2002;14:4267–72. doi:10.1021/cm0203060.
Bohner M. Calcium orthophosphates in medicine: from ceramics to calcium phosphate cements. Injury. 2000;31(S4):D37–47. doi:10.1016/S0020-1383(00)80022-4.
Brown WE, Chow LC. Dental restorative cement pastes. US Patent No. 4518430; 1985.
Tyllianakis M, Giannikas D, Panagopoulos A, Panagiotopoulos E, Lambiris E. Use of injectable calcium phosphate in the treatment of intra-articular distal radius fractures. Orthopedics. 2002;25(3):311–5.
Takegami K, Sano T, Wakabayashi H, Sonoda J, Yamazaki T, Morita S, Shibuya T, Uchida A. New ferromagnetic bone cement for local hyperthermia. J Biomed Mater Res. 1998;43:210–4. doi:10.1002/(SICI)1097-4636(199822)43:2<210:AID-JBM16>3.0.CO;2-L.
Heini PF, Berlemann U. Bone substitutes in vertebroplasty. Eur Spine J. 2001;10:S203–15. doi:10.1007/s005860100308.
Phillips FM. Minimally invasive treatments of osteoporotic vertebral compression fractures. Spine. 2003;28:S45–53.
Lewis G. Injectable bone cements for use in vertebroplasty and kyphoplasty: state of the art review. J Biomed Mater Res. 2005;76B(2):456–68. doi:10.1002/jbm.b.30398.
Belkoff SM, Mathis JM, Jasper LE. Ex vivo biomechanical comparison of hydroxyapatite and polymethylmethacrylate cements for use with vertebroplasty. Am J Neuroradiol. 2002;23:1647–51.
Bohner M. Physical and chemical aspects of calcium phosphates used in spinal surgery. Eur Spine J. 2001;10:S114–21. doi:10.1007/s005860100276.
Fernández E, Vlad MD, Gel MM, López J, Torres R, Cauich JV, Bohner M. Modulation of porosity in apatitic cements by the use of α-tricalcium phosphate–calcium sulphate dihydrate mixtures. Biomaterials. 2005;26:3395–404. doi:10.1016/j.biomaterials.2004.09.023.
Gisep A, Wieling R, Bohner M, Matter S, Schneider E, Rahn B. Resorption patterns of calcium–phosphate cements in bone. J Biomed Mater Res. 2003;66:532–40. doi:10.1002/jbm.a.10593.
Carrodeguas RG, De Aza AH, Turrillas X, Pena P, De Aza S. New approach to the β → α polymorphic transformation in magnesium-substituted tricalcium phosphate and its practical implications. J Am Ceram Soc. 2008;91:1281–6. doi:10.1111/j.1551-2916.2008.02294.x.
Reid JW, Tuck L, Sayer M, Fargo K, Hendry JA. Synthesis and characterization of single-phase silicon-substituted α-tricalcium phosphate. Biomaterials. 2006;27(15):2916–25. doi:10.1016/j.biomaterials.2006.01.007.
Cicek G, Aksoy EA, Durucan C, Hasirci N. Alpha-tricalcium phosphate (α-TCP): solid state synthesis from different calcium precursors and the hydraulic reactivity. J Mater Sci Mater Med. 2011;22:809–17. doi:10.1007/s10856-011-4283-x.
Lilley KJ, Gbureck U, Knowles JC, Farrar DF, Barralet JE. Cement from magnesium substituted hydroxyapatite. J Mater Sci Mater Med. 2005;16(5):455–60. doi:10.1007/s10856-005-6986-3.
Kannan S, Rocha JHG, Ventura JMG, Lemos AF, Ferreira JMF. Effect of Ca/P ratio of precursors on the formation of different calcium apatitic ceramics—an X-ray diffraction study. Scripta Mater. 2005;53:1259–62. doi:10.1016/j.scriptamat.2005.07.037.
Lilley KJ, Gburech U, Wright AJ, Farrar DF, Barralet JE. Cement from nanocrystalline hydroxyapatite: effect of calcium phosphate ratio. J Mater Sci Mater Med. 2005;16:1185–90. doi:10.1007/s10856-005-4727-2.
Wang H, Lee J-K, Moursi A, Lannutti JJ. Ca/P ratio effects on the degradation of hydroxyapatite in vitro. J Biomed Mater Res. 2003;67A:599–608. doi:10.1002/jbm.a.10538.
Burguera EF, Guitián F, Chow LC. Effect of the calcium to phosphate ratio of tetracalcium phosphate on the properties of calcium phosphate bone cement. J Biomed Mater Res. 2008;85A:674–83. doi:10.1002/jbm.a.31478.
Standard Test Method: ASTM C266-89. Time of setting of hydraulic cement paste by Gillmore needles. In: Annual book of ASTM standards, vol. 04.01. Cement, lime, gypsum. Philadelphia: ASTM; 1993. p. 444–472.
Carrodeguas RG, De Aza S. α-Tricalcium phosphate: synthesis, properties and biomedical applications. Acta Biomater. 2011;7(10):3536–46. doi:10.1016/j.actbio.2011.06.019.
Fernández E, Gil FJ, Ginebra MP, Driessens FCM, Planell JA, Best SM. Calcium phosphate bone cements for clinical applications. I: solution chemistry. J Mater Sci Mater Med. 1999;10:169–76. doi:10.1023/A:1008937507714.
Fernández E, Gil FJ, Ginebra MP, Driessens FCM, Planell JA, Best SM. Calcium phosphate bone cements for clinical applications. II: precipitate formation during setting reactions. J Mater Sci Mater Med. 1999;10:177–83. doi:10.1023/A:1008989525461.
Francis MD. The inhibition of calcium hydroxyapatite crystal growth by polyphosphonates and polyphosphates. Calcif Tissue Int. 1969;3(1):151–62. doi:10.1007/BF02058658.
Fernández E, Ginebra MP, Boltong MG, Driessens FCM, Ginebra J, De Maeyer EA, Verbeeck RM, Planell JA. Kinetic study of the setting reaction of calcium phosphate bone cements. J Biomed Mater Res. 1996;32:367–74. doi:10.1002/jbm.10264.
Acknowledgments
The authors thank funding through project MAT2010-19431 (Ministerio de Ciencia e Innovación, Spain). Special thanks to Ms. Maria Odriozola for the English grammar corrections.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vlad, M.D., Gómez, S., Barracó, M. et al. Effect of the calcium to phosphorus ratio on the setting properties of calcium phosphate bone cements. J Mater Sci: Mater Med 23, 2081–2090 (2012). https://doi.org/10.1007/s10856-012-4686-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4686-3