Abstract
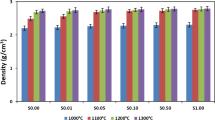
Synthetic calcium phosphate ceramics as β-tricalcium phosphate (Ca3(PO4)2; β-TCP) are currently successfully used in human bone surgery. The aim of this work was to evaluate the influence of the presence of sodium ion in β-TCP on its mechanical and biological properties. Five Na-doped-β-TCP [Ca10.5−x/2Na x (PO4)7, 0 ≤ x ≤ 1] microporous pellets were prepared via solid phase synthesis, and their physico-chemical data (lattice compacity, density, porosity, compressive strength, infrared spectra) denote an increase of the mechanical properties and a decrease of the solubility when the sodium content is raised. On the other hand, the in vitro study of MC3T3-E1 cell activity (morphology, MTS assay and ALP activity) shows that the incorporation of sodium does not modify the bioactivity of the β-TCP. These results strongly suggest that Na-doped-β-TCP appear to be good candidates for their use as bone substitutes.









Similar content being viewed by others
References
LeGeros JP. Biodegradation and bioresorption of calcium phosphate ceramics. Clin Mater. 1993;14:65–88.
Cavagna R, Daculsi G, Bouler J. Macroporous calcium phosphate ceramic: a prospective study of 106 cases in lumbar spinal fusion. J Long Term Eff Med Implants. 1999;9:403–12.
Ransford A, Morley T, Edgar M, Webb P, Passuti N, Chopin D, et al. Synthetic porous ceramic compared with autograft in scoliosis surgery. A prospective, randomized study of 341 patients. J Bone Joint Surg Br. 1998;80:13–8.
Rey C. Calcium phosphate biomaterials and bone mineral. Differences in composition, structures and properties. Biomaterials. 1990;11:13–5.
Bouler J-M, Trécant M, Delécrin J, Royer J, Passuti N, Daculsi G. Macroporous biphasic calcium phosphate ceramics: influence of five synthesis parameters on compressive strength. J Biomed Mater Res. 1996;32:603–9.
Pecqueux F, Tancret F, Payraudeau N, Bouler J-M. Influence of microporosity and macroporosity on the mechanical properties of biphasic calcium phosphate bioceramics: modelling and experiment. J Eur Ceram Soc. 2010;30:819–29.
Perera FH, Martinez-Vazquez FJ, Miranda P, Ortiz AL, Pajares A. Clarifying the effect of sintering conditions on the microstructure and mechanical properties of beta-tricalcium phosphate. Ceram Int. 2010;36(6):1929–35.
Kawamura H, Ito A, Miyakawa S, Layrolle P, Ojima K, Ichinose N, et al. Stimulatory effect of zinc-releasing calcium phosphate implant on bone formation in rabbit femora. J Biomed Mater Res. 2000;50(2):184–90.
Ito A, Kawamura H, Miyakawa S, Layrolle P, Kanzaki N, Treboux G, et al. Resorbability and solubility of zinc-containing tricalcium phosphate. J Biomed Mater Res. 2002;60:224–31.
Lazoryak BI, Strunenkova TV, Golubev VN, Vovk EA, Ivanov LN. Triple phosphates of calcium, sodium and trivalent elements with whitlockite-like structure. Mater Res Bull. 1996;31(2):207–16.
Obadia L, Deniard D, Alonso B, Rouillon T, Jobic S, Guicheux J, et al. Effect of sodium doping in β-tricalcium phosphate on its structure and properties. Chem Mater. 2006;18:1425–33.
Tabor D. The hardness of solids. Rev Phys Technol. 1970;1:145–79.
Shannon R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A. 1976;32(5):751–67.
Rodriguez-Carvajal J, Roisnel T. Fullprof.98 and WinPLOTR: New Windows 95/NT application for diffraction. Commission for powder diffraction, international union of crystallography 1998; newsletter n 20.
Rodriguez-Lorenzo LM, Hart JN, Gross KA. Influence of fluorine in the synthesis of apatites. Synthesis of solid solutions of hydroxy-fluorapatite. Biomaterials. 2003;24(21):3777–85.
Berar JF, Lelann P. E.S.D’s and estimated probalble error obtained in Rietveld refinement with local correlation. J Appl Cryst. 1991;24:1–5.
Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J. Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res. 2003;18(11):2060–8.
Suzuki A, Ghayor C, Guicheux J, Magne D, Quillard S, Kakita A, et al. Enhanced expression of the inorganic phosphate transporter Pit-1 is involved in BMP-2-induced matrix mineralization in osteoblast-like cells. J Bone Miner Res. 2006;21(5):674–83.
Citeau A, Guicheux J, Vinatier C, Layrolle P, Nguyen TP, Pilet P, et al. In vitro biological effects of titanium rough surface obtained by calcium phosphate grid blasting. Biomaterials. 2005;26(2):157–65.
Julien M, Khairoun I, LeGeros RZ, Delplace S, Pilet P, Weiss P, et al. Physico-chemical–mechanical and in vitro biological properties of calcium phosphate cements with doped amorphous calcium phosphates. Biomaterials. 2007;28:956–65.
Relic B, Guicheux J, Mezin F, Lubberts E, Togninalli D, Garcia I, et al. Il-4 and IL-13, but not IL-10, protect human synoviocytes from apoptosis. J Immunol. 2001;166(4):2775–82.
Verron E, Masson M, Khoshniat S, Duplomb L, Wittrant Y, Baud’huin M, et al. Gallium modulates osteoclastic bone resorption in vitro without affecting osteoblasts. Br J Pharmacol. 2010;159:1681–92.
Quillard S, Obadia L, Deniard P, Bujoli B, Bouler JM. Vibrational properties of sodium substituted β-tricalcium phosphate. Key Eng Mater. 2008;361–363:75–8.
Rey C, Shimizu M, Collins B, Glimcher MJ. Resolution-enhanced Fourier transform infrared spectroscopy study of the environment of phosphate ions in the early deposits of a solid phase of calcium-phosphate in bone and enamel, and their evolution with age. I: Investigations in the ν4 PO4 domain. Calcif Tissue Int. 1990;46:384–94.
Rey C, Shimizu M, Collins B, Glimcher MJ. Resolution-enhanced Fourier transform infrared spectroscopy study of the environment of phosphate ions in the early deposits of a solid phase of calcium-phosphate in bone and enamel, and their evolution with age. I: Investigations in the ν3 PO4 domain. Calcif Tissue Int. 1991;49:383–8.
Elliott JC. Structure and chemistry of the apatites and other calcium phosphates. Studies in inorganic chemistry. Amsterdam: Elsevier; 1994. p. 389.
LeGeros RZ. Calcium phosphate in oral biology and medicine. In: Myers HM, editor. Monograph in oral science. Switzerland: Karger; 1991. p. 1–201.
Collin I, Lamy B, Gauthier O, Bouler J-M. Improvement of macroporous biphasic phosphocalcic ceramics for the filling of bone defects. ITBM-RBM. 2005;26(4):247–8.
Gauthier O, Muller R, von Stechow D, Lamy B, Weiss P, Bouler J-M, et al. In vivo bone regeneration with injectable calcium phosphate biomaterial: a three-dimensional micro-computed tomographic, biomechanical and SEM study. Biomaterials. 2005;26(27):5444–53.
Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21(7):667–81.
Boyan BD, Schwartz Z, Lohmann CH, Sylvia VL, Cochran DL, Dean DD, et al. Pretreatment of bone with osteoclasts affects phenotypic expression of osteoblast-like cells. J Orthop Res. 2003;21(4):638–47.
Bouler J-M, Daculsi G. In vitro carbonated apatite precipitation on biphasic calcium phosphate pellets presenting various HA/-TCP ratios. Key Eng Mater. 2001;192–195:119–22.
Kokubo T, Kim HM, Kawashita M, Nakamura T. Process of calcification on artificial materials. Z Kardiol. 2001;90(3):86–91.
Xavier SP, Carvalho PS, Beloti MM, Rosa AL. Response of rat bone marrow cells to commercially pure titanium submitted to different surface treatments. J Dent. 2003;31(3):173–80.
Chesmel KD, Clark CC, Brighton CT, Black J. Cellular responses to chemical and morphologic aspects of biomaterial surfaces. II. The biosynthetic and migratory response of bone cell populations. J Biomed Mater Res. 1995;29(9):1101–10.
Deligianni DD, Katsala ND, Koutsoukos PG, Missirlis YF. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials. 2001;22(1):87–96.
Maeno S, Niki Y, Matsumoto H, Morioka H, Yatabe T, Funayama A, et al. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials. 2005;26(23):4847–55.
Meleti Z, Shapiro IM, Adams CS. Inorganic phosphate induces apoptosis of osteoblast-like cells in culture. Bone. 2000;27(3):359–66.
Farley JR, Hall SL, Tanner MA, Wergedal JE. Specific activity of skeletal alkaline phosphatase in human osteoblast-line cells regulated by phosphate, phosphate esters, and phosphate analogs and release of alkaline phosphatase activity inversely regulated by calcium. J Bone Miner Res. 1994;9(4):497–508.
Acknowledgment
This work was supported by CNRS “Programme Matériaux Nouveaux, Nouvelles Fonctionnalités”, Région Pays de Loire “Programme Biomatériaux S3” and Fondation Avenir pour la Recherche Médicale appliquée, étude ET2-321. Marion Julien received a fellowship from INSERM and region des Pays de la Loire.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obadia, L., Julien, M., Quillard, S. et al. Na-doped β-tricalcium phosphate: physico-chemical and in vitro biological properties. J Mater Sci: Mater Med 22, 593–600 (2011). https://doi.org/10.1007/s10856-010-4219-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-010-4219-x