Abstract
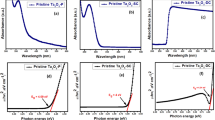
Synthesis of energy efficient materials is the integral step towards tackling global energy crises in the current era. Present work elucidates the synthesis, characterization, and energy related applications of the lanthanides tri-doped zirconium semiconductor system comprising of Ln3+ co-doped ZrO2 (Ln3+ = Ce3+, Pr3+, and Nd3+). Synthesis has been done by adopting 5% doping strategy following chemical co-precipitation route. Precursors and thin films have been characterized via UV–Vis, FT-IR, XRD, and FE-SEM analysis. This material possessed a bandgap energy ranging between 3.6 and 4 eV and Baddeleyite monoclinic phase with 60 nm crystallite size exhibiting P21/c space group with the Zr4+ bonded with seven O2− atoms leading to formation of pentagonal bipyramids of ZrO7. Thin films of Ln3+ co-doped ZrO2 were marked by profound smoothness and maximum surface coverage. The scaffolding performance of the of Ln3+ co-doped ZrO2 was investigated in cesium lead halide perovskite solar cell device, which excelled in gaining an efficiency of 14.1% with the 66% of fill factor. Synthesized material was also explored for electrical charge storage for supercapacitor application by decorating 80% of it on the nickel form current collector (area: 1 × 1 cm2 and thickness: ~0.7 mm). The specific capacitance of this material exceeded the conventionally used materials by reaching up to 350.6 F g−1 making it a potential electrode material with the stabilized electrochemical performance using 0.1 M NaCl as a supporting electrolyte. Impedance studies in this regard indicated faster reaction kinetics and lower smaller series resistance (Rs) of 1.9 Ω. Finally, this material was employed as a bifunctional electro-catalyst for oxygen and hydrogen evolution. With the lowest overpotential and Tafel slope values of 133 mV and 118.9 mV dec−1, the developed electro-catalyst expressed more affinity as an HER electro-catalyst with the Volmer–Heyrovský mechanistic pathway for hydrogen generation. Voltammteric, potentiometric, and amperometric electro-analyses exhibited the excellent durability and service life for 100 min inside 0.1 M alkaline electrolyte of the developed semiconductor material which can be commercialized after optimization.







Similar content being viewed by others
Data availability
Data will be made available on request.
Code availability
Not applicable.
References
T. Zahra, K.S. Ahmad, C. Zequine, R. Gupta, A. Thomas, M.A. Malik, S. Iram, D. Ali, Biomimmetic ZrO2@PdO nanocompsites: fabrication, characterization, and water splitting potential exploration. J. Int. Energy Res. 46, 8516–8526 (2022). https://doi.org/10.1002/er.7736
T. Zahra, K.S. Ahmad, C. Zequine, R.K. Gupta, A.G. Thomas, M.A. Malik, S.B. Jaffri, D. Ali, Electro-catalyst [ZrO2/ZnO/PdO]-NPs green functionalization: fabrication, characterization and water splitting potential assessment. J. Int. Hydrog. Energy 46, 19347–19362 (2021). https://doi.org/10.1016/j.ijhydene.2021.03.094
H. Aydın, U. Kurtan, M. Demir, S. Karakuş, Synthesis and application of a self-standing zirconia-based carbon nanofiber in a supercapacitor. Energy Fuels 36, 2212–2219 (2022). https://doi.org/10.1021/acs.energyfuels.1c04208
A. Ćirić, S. Stojadinović, The spectroscopic quality factor, phase, morphological, structural, and photoluminescent analysis of ZrO2: Nd3+ coatings created by Plasma electrolytic oxidation. J. Lum. 1, 119665 (2023). https://doi.org/10.1016/j.jlumin.2022.119665
C. Salas-Juárez, S.E. Burruel-Ibarra, M.I. Gil-Tolano, A.P. Rodriguez, F. Romo-Garcia, A.R. Garcia-Haro, F. Brown, M. Yacaman-Valdez, J.L. Iriqui-Razcón, M. Martínez-Gil, R. Melendrez, Persistent luminescence of ZrO2: Tb3+ after beta particle irradiation for dosimetry applications. J. Lum. 16, 119712 (2023). https://doi.org/10.1016/j.jlumin.2023.119712
S. Yilmaz, S. Cobaner, E. Yalaz, B. Amini Horri, Synthesis and characterization of gadolinium-doped zirconia as a potential electrolyte for solid oxide fuel cells. Energies 15, 2826 (2022). https://doi.org/10.3390/en15082826
Y. Samantaray, D.J. Martin, R.G. Agarwal, N.J. Gibson, J.M. Mayer, Proton-coupled electron transfer of cerium oxide nanoparticle thin-film electrodes. J. Phys. Chem. C (2023). https://doi.org/10.1021/acs.jpcc.2c06783
S.B. Jaffri, K.S. Ahmad, K.H. Thebo, F. Rehman, Recent developments in carbon nanotubes-based perovskite solar cells with boosted efficiency and stability. Zeit für Phys. Chem. 235, 1539–1572 (2021). https://doi.org/10.1515/zpch-2020-1729
S.B. Jaffri, K.S. Ahmad, Interfacial engineering revolutionizers: perovskite nanocrystals and quantum dots accentuated performance enhancement in perovskite solar cells. Crit. Rev. Solid State Mater. Sci. 46, 251–279 (2021). https://doi.org/10.1080/10408436.2020.1758627
S. Lee, J.Y. Kim, K.S. Hong, H.S. Jung, J.K. Lee, H. Shin, Enhancement of the photoelectric performance of dye-sensitized solar cells by using a CaCO3-coated TiO2 nanoparticle film as an electrode. Sol. Energy Mater. Sol. Cells 90, 2405–2412 (2006). https://doi.org/10.1016/j.solmat.2006.03.013
A. Kulkarni, A.K. Jena, H.W. Chen, Y. Sanehira, M. Ikegami, T. Miyasaka, Revealing and reducing the possible recombination loss within TiO2 compact layer by incorporating MgO layer in perovskite solar cells. Sol. Energy 136, 379–384 (2016). https://doi.org/10.1016/j.solener.2016.07.019
T.M. Abdel-Fattah, S. Ebrahim, K. Gasmalla, M. Soliman, The impacts of zirconia as a spacer layer on fill factor of hole-free perovskite solar cells. In Electrochemical Society Meeting Abstracts aimes. Electrochem. Soc. 59, 2159–2159 (2018). https://doi.org/10.1149/MA2018-02/59/2159
M. Che, L. Zhu, Y.L. Zhao, D.S. Yao, X.Q. Gu, J. Song, Y.H. Qiang, Enhancing current density of perovskite solar cells using TiO2-ZrO2 composite scaffold layer. Mater. Sci. Semicond. Proc. 56, 29–36 (2016). https://doi.org/10.1016/j.mssp.2016.07.003
Y. Zhao, J. Wei, H. Li, Y. Yan, W. Zhou, D. Yu, Q. Zhao, A polymer scaffold for self-healing perovskite solar cells. Nat. Commun. 7, 10228 (2016). https://doi.org/10.1038/ncomms10228
I. Shaheen, K.S. Ahmad, S.B. Jaffri, D. Ali, Biomimetic [MoO3@ZnO] semiconducting nanocomposites: chemo-proportional fabrication, characterization and energy storage potential exploration. Renew. Energy 167, 568–579 (2021). https://doi.org/10.1016/j.renene.2020.11.115
M.N. Ashiq, A. Aman, T. Alshahrani, M. Faisal Iqbal, A. Razzaq, M. Najam-Ul-Haq, A. Shah, J. Nisar, D. Tyagi, M. Fahad Ehsan, Enhanced electrochemical properties of silver-coated zirconia nanoparticles for supercapacitor application. J. Taibah Univ. Sci. 15, 10–16 (2021). https://doi.org/10.1080/16583655.2020.1867338
Z. Pang, J. Duan, Y. Zhao, Q. Tang, B. He, L. Yu, A ceramic NiO/ZrO2 separator for high-temperature supercapacitor up to 140° C. J. Power Sources 400, 126–134 (2018). https://doi.org/10.1016/j.jpowsour.2018.08.008
C.V. Reddy, R. Koutavarapu, J. Shim, B. Cheolho, K.R. Reddy, Novel g-C3N4/Cu-doped ZrO2 hybrid heterostructures for efficient photocatalytic Cr (VI) photoreduction and electrochemical energy storage applications. Chemosphere 295, 133851 (2022). https://doi.org/10.1016/j.chemosphere.2022.133851
S. Srinivasan, C. Vivek, P. Sakthivel, G. Chamundeeswari, S.P. Bharathi, S. Amuthameena, B. Balraj, Synthesis of Ag incorporated ZrO2 nanomaterials for enhanced electrochemical energy storage applications. Inorg. Chem. Commun. 138, 109262 (2022). https://doi.org/10.1016/j.inoche.2022.109262
I. Shaheen, I. Hussain, T. Zahra, R. Memon, A.A. Alothman, M. Ouladsmane, A. Qureshi, J.H. Niazi, Electrophoretic deposition of ZnO/CuO and ZnO/CuO/rGO heterostructure based film as environmental Benign flexible electrode for supercapacitor. Chemosphere 18, 138149 (2023). https://doi.org/10.1016/j.chemosphere.2023.138149
N. Abbas, I. Shaheen, I. Ali, M. Ahmad, S.A. Khan, A. Qureshi, J.H. Niazi, M. Imran, C. Lamiel, M.Z. Ansari, I. Hussain, Effect of growth duration of Zn0.76Co0.24S interconnected nanosheets for high-performance flexible energy storage electrode materials. Ceram. Int. 48, 34251–34257 (2022). https://doi.org/10.1016/j.ceramint.2022.07.225
S.M. Hassan, M.T. Siddique, M. Fakhar-e-Alam, M. Atif, A. Saifullah, N. Marwat, A. Khurshid, O. Noor, N. Hossain, S. Ahmad, K.S. Alimgeer, Hydrothermally synthesized lanthanide-incorporated multifunctional zirconia nanoparticles: potential candidate for multimodal imaging. J. King Saud Univ. Sci. 34, 102080 (2022). https://doi.org/10.1016/j.jksus.2022.102080
M. Ijaz, Plasmonic hot electrons: Potential candidates for improved photocatalytic hydrogen production. J. Int. Hydrog. Energy (2022). https://doi.org/10.1016/j.ijhydene.2022.11.251
T. Iqbal, A. Hassan, M. Ijaz, M. Salim, M. Farooq, M. Zafar, M.B. Tahir, Chromium incorporated copper vanadate nano-materials for hydrogen evolution by water splitting. Appl. Nanosci. 11, 1661–1671 (2021). https://doi.org/10.1007/s13204-021-01786-8
M.B. Tahir, M. Sagir, S. Muhammad, S.M. Siddeeg, T. Iqbal, A.M. Asiri, M. Ijaz, Hierarchical WO3@BiVO4 nanostructures for improved green energy production. Appl. Nanosci. 10, 1183–1190 (2020). https://doi.org/10.1007/s13204-019-01180-5
D. Strmcnik, P.P. Lopes, B. Genorio, V.R. Stamenkovic, N.M. Markovic, Design principles for hydrogen evolution reaction catalyst materials. Nano Energy 29, 29–36 (2016). https://doi.org/10.1016/j.nanoen.2016.04.017
N. Han, K.R. Yang, Z. Lu, Y. Li, W. Xu, T. Gao, Z. Cai, Y. Zhang, V.S. Batista, W. Liu, X. Sun, Nitrogen-doped tungsten carbide nanoarray as an efficient bifunctional electrocatalyst for water splitting in acid. Nat. Commun. 9, 924 (2018). https://doi.org/10.1038/s41467-018-03429-z
H. Sun, X. Xu, Z. Yan, X. Chen, L. Jiao, F. Cheng, J. Chen, Superhydrophilic amorphous Co–B–P nanosheet electrocatalysts with Pt-like activity and durability for the hydrogen evolution reaction. J. Mater. Chem. A 6, 22062–22069 (2018). https://doi.org/10.1039/C8TA02999G
V.S. Thoi, Y. Sun, J.R. Long, C.J. Chang, Complexes of earth-abundant metals for catalytic electrochemical hydrogen generation under aqueous conditions. Chem. Soc. Rev. 42, 2388–2400 (2013). https://doi.org/10.1039/C2CS35272A
D. Bogachuk, J. Girard, S. Tilala, D. Martineau, S. Narbey, A. Verma, A. Hinsch, M. Kohlstädt, L. Wagner, Nanoarchitectonics in fully printed perovskite solar cells with carbon-based electrodes. Nanoscale 1, 1–10 (2023). https://doi.org/10.1039/D2NR05856A
W. Jinxi, W. Aimin, A.K. Ghasemi, M.S. Lashkenari, E. Pashai, C. Karaman, D.E. Niculina, H. Karimi-Maleh, Tailoring of ZnFe2O4-ZrO2-based nanoarchitectures catalyst for supercapacitor electrode material and methanol oxidation reaction. Fuel 334, 126685 (2023). https://doi.org/10.1016/j.fuel.2022.126685
M. Beke, T. Velempini, K. Pillay, Synthesis and application of NiO-ZrO2@ g-C3N4 nanocomposite for high-performance hybrid capacitive deionisation. Result Chem. 5, 100799 (2023). https://doi.org/10.1016/j.rechem.2023.100799
C. Lee, K. Shin, Y. Park, Y.H. Yun, G. Doo, G.H. Jung, M. Kim, W.C. Cho, C.H. Kim, H.M. Lee, H.Y. Kim, Catalyst-support interactions in Zr2ON2-supported IrOx electrocatalysts to break the trade-off relationship between the activity and stability in the acidic oxygen evolution reaction. Adv. Func. Mater. 1, 2301557 (2023). https://doi.org/10.1002/adfm.202301557
R. Palani, Y.S. Wu, S.H. Wu, R. Jose, C.C. Yang, Metal-organic framework-derived ZrO2/NiCo2O4/graphene mesoporous cake-like structure as enhanced bifunctional electrocatalytic cathodes for long life Li-O2 batteries. Electrochim. Acta 412, 140147 (2022). https://doi.org/10.1016/j.electacta.2022.140147
Q. Mohsen, W.S. Al-Gethami, Z. Zaki, S.H. Alotaibi, M.M. Ibrahim, M. Ezzat, M.A. Amin, M.M. Kamel, N.Y. Mostafa, Effect of pH on hydrothermal synthesis of ZrO2 nanoparticles and their electrocatalytic activity for hydrogen production. Int. J. Electrochem. Sci. 17, 2 (2022). https://doi.org/10.20964/2022.07.24
S. Xu, X. Tan, F. Liu, L. Zhang, Y. Huang, B.A. Goodman, W. Deng, Growth and optical properties of thulia-doped cubic yttria stabilized zirconia single crystals. Ceram. Int. 45, 15974–15979 (2019). https://doi.org/10.1016/j.ceramint.2019.05.106
C. Dhandapani, R. Narayanasamy, S.N. Karthick, K.V. Hemalatha, S. Selvam, P. Hemalatha, S.D. Kirupha, H.J. Kim, Drastic photocatalytic degradation of methylene blue dye by neodymium doped zirconium oxide as photocatalyst under visible light irradiation. Optik 127, 10288–10296 (2016). https://doi.org/10.1016/j.ijleo.2016.08.048
Y. Li, J. Wang, Z. Huang, C. Qian, Y. Tian, Y. Duan, An Eu-doped Zr-metal-organic framework for simultaneous detection and removal of antibiotic tetracycline. J. Environ. Chem. Eng. 9, 106012 (2021). https://doi.org/10.1016/j.jece.2021.106012
Q. Yang, H. Hong, Y. Luo, Heterogeneous nucleation and synthesis of carbon dots hybrid Zr-based MOFs for simultaneous recognition and effective removal of tetracycline. J. Chem. Eng. 392, 123680 (2020). https://doi.org/10.1016/j.cej.2019.123680
T. Zahra, K.S. Ahmad, C. Zequine, R. Gupta, M.A. Malik, J.H. Niazi, A. Qureshi, Bio-inspired NiO/ZrO2 mixed oxides (NZMO) for oxygen evolution reactions: from facile synthesis to electrochemical analysis. J. Chem. Technol. Biotechnol. 98, 296–305 (2023). https://doi.org/10.1002/jctb.7246
B. Murali Babu, S. Vadivel, High performance humidity sensing properties of indium tin oxide (ITO) thin films by sol–gel spin coating method. J. Mater. Sci. 28, 2442–2447 (2017). https://doi.org/10.1007/s10854-016-5816-3
M. Fang, A. Aristov, K.V. Rao, A.V. Kabashin, L. Belova, Particle-free inkjet printing of nanostructured porous indium tin oxide thin films. RSC Adv. 3, 19501–19507 (2013). https://doi.org/10.1039/C3RA40487K
O. Dimitrov, I. Stambolova, T. Babeva, K. Lazarova, G. Avdeev, M. Shipochka, R. Mladenova, S. Simeonova, High intensity orange-red emission of chemically deposited Sm3+-doped ZrO2 thin films-beneficial effects of host and dopant. J. Mater. Res. Technol. 18, 3026–3034 (2022). https://doi.org/10.1016/j.jmrt.2022.04.013
A. Ćirić, S. Stojadinović, Photoluminescence studies of ZrO2: Tm3+/Yb3+ coatings formed by plasma electrolytic oxidation. J. Lum. 214, 116568 (2019). https://doi.org/10.1016/j.jlumin.2019.116568
A. Ćirić, S. Stojadinović, Photoluminescence of ZrO2: Gd3+ and ZrO2: Dy3+ coatings formed by the plasma electrolytic oxidation. J. Alloys Compds. 832, 154907 (2020). https://doi.org/10.1016/j.jallcom.2020.154907
A. Zafar, K.S. Ahmad, S.B. Jaffri, M. Sohail, Physical vapor deposition of SnS: PbS-dithiocarbamate chalcogenide semiconductor thin films: elucidation of optoelectronic and electrochemical features. Phosphorus Sulfur Silicon Relat Elem 196, 36–46 (2020). https://doi.org/10.1080/10426507.2020.1799371
H. Siraj, K.S. Ahmad, S.B. Jaffri, M. Sohail, Synthesis, characterization and electrochemical investigation of physical vapor deposited barium sulphide doped iron sulphide dithiocarbamate thin films. Microelectron. Eng. 233, 111400 (2020). https://doi.org/10.1016/j.mee.2020.111400
K.S. Ahmad, S.N. Naqvi, S.B. Jaffri, Systematic review elucidating the generations and classifications of solar cells contributing towards environmental sustainability integration. Rev. Inorg. Chem. 41, 21–39 (2021). https://doi.org/10.1515/revic-2020-0009
S.B. Jaffri, K.S. Ahmad, Newfangled progressions in the charge transport layers impacting the stability and efficiency of perovskite solar cells. Rev. Inorg. Chem. 42, 137–159 (2022). https://doi.org/10.1515/revic-2021-0004
M.F. Vildanova, A.B. Nikolskaia, S.S. Kozlov, O.I. Shevaleevskiy, O.V. Almjasheva, V.V. Gusarov, Group IV oxides for perovskite solar cells. Doklady Phys. Chem. 496, 13–19 (2021). https://doi.org/10.1134/S0012501621020020
Y. Li, L. Zhao, S. Wei, M. Xiao, B. Dong, L. Wan, S. Wang, Effect of ZrO2 film thickness on the photoelectric properties of mixed-cation perovskite solar cells. Appl. Surf. Sci. 439, 506–515 (2018). https://doi.org/10.1016/j.apsusc.2018.01.005
Z. Meng, D. Guo, J. Yu, K. Fan, Investigation of Al2O3 and ZrO2 spacer layers for fully printable and hole-conductor-free mesoscopic perovskite solar cells. Appl. Surf. Sci. 430, 632–638 (2018). https://doi.org/10.1016/j.apsusc.2017.05.018
J.W. Lee, S.G. Kim, S.H. Bae, D.K. Lee, O. Lin, Y. Yang, N.G. Park, The interplay between trap density and hysteresis in planar heterojunction perovskite solar cells. Nano Lett. 17, 4270–4276 (2017). https://doi.org/10.1021/acs.nanolett.7b01211
C.H. Hsu, K.T. Chen, L.Y. Lin, W.Y. Wu, L.S. Liang, P. Gao, Y. Qiu, X.Y. Zhang, P.H. Huang, S.Y. Lien, W.Z. Zhu, Tantalum-doped TiO2 prepared by atomic layer deposition and its application in perovskite solar cells. Nanomaterials 11, 1504 (2021). https://doi.org/10.3390/nano11061504
D.P. Shoemaker, S.A. Corr, R. Seshadri, Porosity through reduction in metal oxides. MRS Online Proc. Lib. 1148, 1–8 (2008). https://doi.org/10.1557/PROC-1148-PP10-01
J. Anupriya, N. Karuppusamy, T.W. Chen, S.M. Chen, K. Balamurugan, M. Akilarasan, X. Liu, J. Yu, Enhancing catalytic activity through the construction of praseodymium tungstate decorated on hierarchical three-dimensional porous biocarbon for determination of furazolidone in aquatic samples. Chemosphere 313, 137553 (2023). https://doi.org/10.1016/j.chemosphere.2022.137553
W. Zhang, Y. Tan, Y. Gao, J. Wu, B. Tang, Ultrafine nano zirconia as electrochemical pseudocapacitor material. Ceram. Int. 41, 2626–2630 (2015). https://doi.org/10.1016/j.ceramint.2014.10.047
Y.S. Sung, L.Y. Lin, Improving energy storage ability of Universitetet i Oslo-66 as active material of supercapacitor using carbonization and acid treatment. J. Energy Storage 37, 102480 (2021). https://doi.org/10.1016/j.est.2021.102480
A.S. Yasin, I.M. Mohamed, M.T. Amen, N.A. Barakat, C.H. Park, C.S. Kim, Incorporating zirconia nanoparticles into activated carbon as electrode material for capacitive deionization. J. Alloys Compds. 772, 1079–1087 (2019). https://doi.org/10.1016/j.jallcom.2018.09.057
Z. Chang, S. Li, L. Sun, C. Ding, X. An, X. Qian, based electrode comprising zirconium phenylphosphonate modified cellulose fibers and porous polyaniline. Cellulose 26, 6739–6754 (2019). https://doi.org/10.1007/s10570-019-02523-9
Y. Wang, Y. Lu, Z. Hu, J. Sun, G. Xiao, H. Zhao, J. Zhu, Z. Liu, Facile preparation of Zr@carbon electrodes based on polyimide/UiO-66 composites for supercapacitors. Electrochem. Commun. (2023). https://doi.org/10.1016/j.elecom.2023.107449
A.P. Alves, R. Koizumi, A. Samanta, L.D. Machado, A.K. Singh, D.S. Galvao, G.G. Silva, C.S. Tiwary, P.M. Ajayan, One-step electrodeposited 3D-ternary composite of zirconia nanoparticles, rGO and polypyrrole with enhanced supercapacitor performance. Nano Energy 31, 225–232 (2017). https://doi.org/10.1016/j.nanoen.2016.11.018
B. Mete, N.S. Peighambardoust, S. Aydin, E. Sadeghi, U. Aydemir, Metal-substituted zirconium diboride (Zr1-xTMxB2; TM= Ni Co, and Fe) as low-cost and high-performance bifunctional electrocatalyst for water splitting. Electrochim. Acta 389, 138789 (2021). https://doi.org/10.1016/j.electacta.2021.138789
T. Zahra, K.S. Ahmad, Functionalization of Mn2O3/PdO/ZnO electrocatalyst using organic template with accentuated electrochemical potential toward water splitting. J. Int. Energy Res. 46, 452–63 (2022). https://doi.org/10.1002/er.6677
M.M. Gul, K.S. Ahmad, E-beam-deposited Zr2NiS4-GO alloy thin film, a tenacious photocatalyst and efficient electrode for electrical devices. J. Mater. Sci. 57, 7290–7309 (2022). https://doi.org/10.1007/s10853-022-07131-w
B. Sukhbaatar, S. Yoon, B. Yoo, Simple synthesis of a CoO nanoparticle-decorated nitrogen-doped carbon catalyst from spent coffee grounds for alkaline hydrogen evolution. J. Mater. Sci. 57, 18075–21888 (2022). https://doi.org/10.1007/s10853-022-07436-w
Acknowledgements
This research work was supported by Researchers Supporting Project number (RSP2023R100), King Saud University, Riyadh, Saudi Arabia. Authors of this work are highly grateful to the Department of Environmental Sciences, Fatima Jinnah Women University, The Mall, 46000, Rawalpindi, Pakistan, and the Queen Mary University of London, the United Kingdom for providing the technical facilities needed for the completion of this work. Also, the authors want to acknowledge the Higher Education Commission, Pakistan. The concept, idea, and writing of this work are the intellectual property right of Materials and Environmental Chemistry Lab, Lab E-21, Department of Environmental Sciences, Fatima Jinnah Women University, The Mall, 46000, Rawalpindi, Pakistan.
Funding
This research work was supported by Researchers Supporting Project number (RSP2023R100), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
SBJ: Conceptualization, methodology, visualization, data curation, and writing—original draft; KSA: Conceptualization, methodology, supervision, and writing—review & editing; IA: Conceptualization, methodology, supervision, data curation, resources, and writing—review & editing; SMI: resources, and review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jaffri, S.B., Ahmad, K.S., Abrahams, I. et al. Augmented photovoltaic and electrochemical performance of lanthanide (Ln3+ = Ce3+, Pr3+, and Nd3+) doped ZrO2 semiconductor material. J Mater Sci: Mater Electron 34, 1376 (2023). https://doi.org/10.1007/s10854-023-10811-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10811-1