Abstract
Pure and Europium (Eu) doped hydroxyapatite (HAp) powders were prepared by microwave assisted hydrothermal method. A detailed structural examination of the HAp was performed through transmission electron microscopy and X-ray diffraction techniques. Analysis of thermoluminescent (TL) properties of the pure and Eu doped hydroxyapatite by exposing to different dosages of gamma radiation were also conducted. The characterization results show an appreciable influence of the Eu dopant on HAp TL signal, crystal sizes and the proportion of crystalline phases were identified. Rietveld analysis of the XRD patterns confirmed that the dominant hexagonal crystalline phase of pure HAp was diminished and an increase in monoclinic phase was observed accordingly with an increment in the Eu doping. This takes place due to the increment in crystal growth for monoclinic phase and a decrement in the crystal size of hexagonal phase. The thermoluminescence studies showed that Eu doped HAp (0.5 wt%) possess high intensity TL signal and there is a linear relationship between the TL Intensity and gamma radiation dosage in the range from 1.0 to 100 Gy. The results suggest that the Eu doped HAp can be considered as a radiation dosimeter material specifically for gamma radiation.
Similar content being viewed by others
1 Introduction
Hydroxyapatite (HAp) is the main biomineral component found in human hard tissues and its stoichiometry is represented by the chemical formula [Ca10(PO4)6(OH)]. HAp is mainly comprised of calcium and phosphorus, which are in the ratio (Ca/P) of 1.67 for a stochiometric HAp [1]. Nowadays, there is an increasing interest to synthesize HAp in the laboratory due to its bioactivity, similarity in crystallography and chemical composition to the human hard tissue and its uses in different applications like prosthetic, metallic implants, clinical bone augmentation, dental implantology, tumors treatment, cell activation, carrier in drug delivery systems, CO2 gas sensing, catalysis and water treatment [2,3,4,5,6,7,8].
A wide range of chemical synthesis methods were employed utilizing various calcium and phosphate precursors by varying pH, reaction time and calcination temperatures for obtaining HAp nanostructures [9,10,11,12,13,14]. On the other hand, it is possible to increase the field application of synthetic HAp by adding dopants during its synthesis; for example, Iconaru et al. showed that Eu doped HAp presents antimicrobial activity and this property is linearly dependent on the europium concentration [8]. Zheng et al. show that Ln3+ doped hydroxyapatite nanocrystals (Ln = Eu or Tb) presents strong optical properties and could be applied for living cell imaging [15]. HAp has also been used for electron spin resonance dosimetry to quantify gamma radiation dosages for some decades [16].
In addition, pure and doped HAp has interesting thermoluminescence properties when this biomaterial is exposed to ionizing radiation, such as gamma rays [17,18,19,20,21,22]. Thermoluminescence is a thermally stimulated light emission from a semiconductor in a controlled manner after the absorption of energy from different ionising radiation, such as γ-rays, β-rays, X-rays, and UV. The quantity of light emission is always proportional to the dosage received by the material [23,24,25]. Azorin Nieto well explicated the TL phenomenon as follows: Crystalline materials suffers alterations in their structure after the irradiation due to ionization of the crystals; during this process two types of mobile carriers are generated from the crystal lattice: electrons and holes. Both charge carriers are active along the crystal until they are trapped in the crystal defects. Electrons and holes remain trapped until enough energy is supplied to the crystal. Once the necessary energy is supplied to the crystal, electrons and holes are liberated and their recombination releases the excess energy in the form of photons of visible light (luminescence) and the crystal returns to its original state. If this energy is supplied by heating the crystal, then the phenomenon observed is referred as thermally stimulated luminescence or thermoluminescence (TL). TL process involves the evacuation of different traps at different energies liberating the mobile carriers at different temperatures giving rise to a glow curve, which is the characteristic curve of the material and can exhibit one or more peaks depending on the energies required for the charge carrier’s liberation. Importance of TL phenomenon in radiation dosimetry is because the amount of light emitted is proportional to the dosage absorbed by the irradiated material [26].
On the other hand, it is important to point out that the gamma radiation has been applied in several areas of science and technology. For example, it is used in medicine for malignant tumors treatments, sterilization of pharmaceuticals and medical devices, sterilization of healthcare products, preservation of foods and to modify microstructural characteristics of specific materials, amongst many other applications. The dosage radiation varies from some mGy (medical) up to MGy (modification of the matter), depending of the specific application. The energy range characteristic of the gamma radiation begins at about 1000 eV and reaches its upper limit around 30 MeV [27,28,29,30,31,32,33]. Particularly, the study and enhancement of TL properties of pure and doped HAp has attracted attention due to the important applications in medical physics to determine the dosage radiation received by the patients, when gamma radiation is used [25]. Furthermore, a better knowledge of the thermoluminescent behavior of HAp will allow using this biomaterial as a bio dosimeter in retrospective nuclear accidents [34].
In this work, pure and Eu doped HAp powders were synthesized by microwave assisted hydrothermal method to study and understand their thermoluminescent behavior after exposition to gamma ionizing radiation. The main advantages of the process used in this work over conventional-hydrothermal process are: (a) time and energy of the process are reduced due to rapid heat treatment, (b) the kinetics of the reaction are enhanced from one up to two orders of magnitude, (c) the formation of novel phases, and (d) the selective crystallization [35,36,37,38,39,40]. In addition, uniform and homogenous heat generation within the material is achieved by continuous rotation of the specimen, instead of originating from external sources, for microwave hydrothermal process [41, 42]. The effects of the dopant (Eu) on structural, morphological and thermoluminescent properties of the synthesized HAp powders are given in the upcoming sections.
2 Experimental
2.1 Synthesis of pure HAp powders
HAp nanostructures were synthesized utilizing calcium nitrate tetrahydrate (Ca(NO3)2·4H2O—Fisher Scientific grade ACS) and ammonium dihydrogen phosphate ((NH4)2HPO4—Fisher Scientific grade analytical reagent) as precursors, through microwave assisted hydrothermal method. Precursor solutions were prepared by dissolving 11.807 g of (Ca(NO3)2·4H2O) and 6.603 g of (NH4)2HPO4 separately in 50 ml of distilled water as solvent. For obtaining stoichiometric Ca/P molar ratio of 1.67 ml of 0.1 M (NH4)2HPO4 was added to 16.7 ml of 0.1 M Ca(NO3)2·4H2O. The mixed solution was then transferred to a 125 ml Teflon-coated, microwave assisted hydrothermal reactor holding a constant temperature at 200 °C for 40 min at a pH of 9. The reaction mechanism for the formation of hydroxyapatite is explained through Eq. (1) and the complete experimental procedure is resumed in Fig. 1.
2.2 Synthesis of Eu doped HAp powders
Eu doped HAp nanostructures were synthesized by adding 0.1 wt% of Europium(III) nitrate dehydrate in the mixture of 10 ml of 0.1 M (NH4)2HPO4, 16.7 ml of 0.1 M of Ca(NO3)2·4H2O. Later, like the pure HAp synthesis, the mixed solution is transferred to microwave assisted hydrothermal reactor at 200 °C for 40 min at a pH of 9. Similar procedure is followed for 0.5, 0.96 and 2.4 wt% of Eu doped HAP samples (Fig. 1).
Then, both pure and Eu dope HAp powders were recovered from microwave hydrothermal oven and cooled in a dry oven at a temperature of 80 °C until the solid point is reached and were thermally treated at 450 °C for 2 h. Finally, pure and Eu doped HAp powders were characterized for its structural and compositional analysis.
2.3 Characterization
A detailed structural analysis was performed in a JEOL JEM 2010HT transmission electron microscope (TEM) with a resolution of 1.9 Å. Samples were prepared by dispersing the powders in ethanol and depositing a drop on a carbon-coated copper grid. Powder XRD spectra were recorded at room temperature, using a D8 Discover Bruker diffractometer [CuKα = 1.5406 Å radiation source] operating at 40 kV and 40 mA. The diffraction patterns were collected over a 2θ range from 10° to 70° with an incremental step size of 0.04°. The acquisition time was set at 2 s for each scan. For the thermoluminescence analysis, pure and doped hydroxyapatite samples were pressed to obtain pellets of 5 mm in diameter and 1 mm in thickness. Gamma irradiation was conducted using a 60Co-Gammacell (Atomic Energy of Canada Ltd.) at a dosage rate of ≈ 59.77 Gy/h. The total irradiation dosage delivered to the samples was from 1 to 100 dosage Gy. TL readings were made using a Harshaw model 4000 TL Analyzer; glow curves were recorded from 30 to 330 °C at a heating rate of 5 °C/s under a nitrogen atmosphere (Fig. 1).
3 Results and discussion
3.1 TEM analysis
Figure 2a shows a TEM image of pure HAp nanorods obtained by microwave assisted hydrothermal synthesis. The rods have a width ranging from 20 to 40 nm and 60–80 nm length. Figure 2b, c corresponds to TEM images of 0.5 and 0.96 wt% Eu doped HAp, respectively. From Fig. 2 we can observe that the obtained nanorods possess uniform diameters along their length. Eu doping have reduced the HAp nanorod length from 80 to 50 nm (Fig. 2b) for further increase in the Eu doping concentration resulted in the formation of nanorods with length 30 nm and smaller particles around 4–10 nm (Fig. 2c). In general, the dopant remains on the particle surfaces and inhibit the growth [43], which would be reason for reducing the nanorod length, and formation of the HAp nanoparticles at higher Eu wt%. A similar morphology was observed for the remining europium doped HAp samples.
3.2 XRD analysis
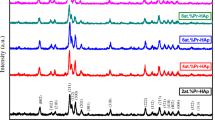
The typical XRD patterns of pure and Eu doped HAp powder are presented in Fig. 3. All the samples revealed the presence of both hexagonal and monoclinic crystalline phases with different quantities. Hexagonal and monoclinic phases obtained are consistent with the phases listed in the JCPDS Cards No. 09-0432 and 76-0694 databases, respectively. The main (h k l) indices for HAp: (100), (101), (200), (002), (102), (210), (211), (112), (300), (202), (310), (222) and (213) are indicated in the Fig. 3. However, for Eu-doped HAp samples some diffraction peaks with lesser intensities located at 14.67, 20.35 and 31.96 and 38.44 in 2-theta scale can be observed (indicated by arrows in Fig. 3); these peaks are associated to europium phosphate hydrate (EuPO4·H2O) with the JCPDS card No. 20-1044.
To determinate the quantity of each crystalline phase and crystal size present in all samples, the Rietveld method was applied using the data obtained from X-ray diffraction patterns, for each sample. Table 1 presents the crystalline parameters calculated for pure and Eu doped HAp. From Table 1 we can observe that the quantity of hexagonal and monoclinic phases for pure HAp are 74.76 and 22.24 wt%, respectively, and the crystal sizes are around 64.37 and 5.7 nm, respectively.
It is evident from Table 1 that the dominant crystalline phase in all samples is hexagonal with relatively larger crystallite size. Also, the hexagonal phase of HAp diminishes while monoclinic phase increases with an increment in the Eu dopant concentration. The monoclinic HAp is relatively more stable phase than hexagonal HAp which makes the dopant (Eu) to easily penetrate into the crystal structure of the hexagonal HAp producing the new crystalline phase EuPO4·H2O [44]. Therefore, the breaking of the hexagonal HAp structure and the subsequent changes in the configurations of OH− ions and other constituents causes the increase in the concentration of the monoclinic structure and decrease in the concentration of hexagonal HAp [45].
3.3 Thermoluminescence properties
The dependence of the TL intensity on the gamma irradiation of the pure and Eu-doped hydroxyapatite with radiation dosage from 1.0 to 100 Gy is shown in Fig. 4; glow curves were recorded for a storage time of 24 h to stabilize the unstable peak at lower temperature. As per Fig. 4a, pure HAp shows a broad and weak thermoluminescence curve that shows a slow growing when the dosage is increasing (Fig. 4a). It is possible to appreciate two maximal peaks located at 130 and 193 °C, mainly for higher dosage radiation, which agrees with different, previously reported works [17, 22, 24]. Zarinfar et al. reported a wide TL curve with a maximal peak located at 200 °C, for HAp samples synthetized by hydrolysis method, thermally treated at 900 °C and irradiated by 60Co gamma radiation with dosage of 100 Gy [17]. Also, Rivera et al. reported a TL signal with two maximal peaks located at 200 and 300 °C for pure HAp when it is exposed to gamma radiation; in that case, HAp was obtained by sol–gel method and thermally treated at 1200 °C; samples thermally treated at 600 and 900 °C showed a very weak TL signal [34]. Besides, Madhukumar et al. reported that there is no TL signal for tricalcium phosphate [Ca3(PO4)2] synthetized by wet precipitation method and irradiated by X-ray, but they confirmed a TL signal for Eu-doped Ca3(PO4)2 [22]. Comparing the present work with previously reported works, the thermoluminescent response of the HAp material varies depending on the synthesis method and the sintering temperature.
The thermoluminescent behavior of Eu-doped HAp (0.1 wt%) was different to pure HAp. In this case, an intense TL signal induced by gamma radiation was observed (Fig. 4b). This signal consists in a well-defined curve with a maximal peak located at 153 °C; the intensity of this peak shows an important increment accordingly with an increase in dosage radiation. There have been only a few scientific reports about the thermoluminescent studies of Eu doped HAp. Zarinfar et al. [17] reported an important influence of Gd dopant on the TL signal induced by gamma radiation in HAp; they found that the TL intensity of HAp: Gd (1%) and HAp: Gd (2%) samples is lesser than pure HAp, but the TL intensity was maximum for 10% of Gd doping in HAp. Madhukumar et al. reported a weak TL signal for Eu-doped tricalcium phosphate [Ca3(PO4)2], when exposed to X-ray. On the other hand, our results show that the nature of electron traps in Eu doped HAp is more effective than pure HAp and the proportion of traps associated with the temperature emission at 130 °C is increasing and slowly displaced to higher energy.
The TL mechanism of HAp: Eu system could be similar to TL mechanism of β-TCP: Dy. The trapping and recombination seems to be characteristic of host lattice. During the γ-irradiation, Eu3+ ions change its valence state to Eu2+ by trapping an electron and the holes released simultaneously might be trapped by the HAp matrix forming radicals derived from PO43−. Some of these defects recombine immediately after creation while other pairs separate by migration and remain as stable defects. During heating the trapped electrons and/or holes will be released and recombine with each other, leading to a TL signal raised because of Eu3+ [22].
As the Eu doping increased up to 0.5 wt%, an intensity peak (located at 130 °C) compared to 0.1 wt% Eu doped HAp can be observed. And its intensity increases accordingly with the dosage radiation, which is corroborated to the proportion of traps associates with the temperature emission at 130 °C [46]; but no displacement of the energy is observed (Fig. 5a). From the Fig. 5a it is also possible to note a second maximum peak at ≈ 270 °C with lower intensity. When Eu dopant in HAp is increased to 0.96 wt%, a substantial change for the thermoluminescent signal of irradiated samples was observed. For this sample, a TL curve with two defined peaks at 142 and 202 °C is observed (Fig. 5b). Although, the TL signal is growing when the dosage radiation is increased, its TL intensity is lesser than 0.1 and 0.5% Eu doped HAp samples.
As the Eu dopant is further increased to 2.4 wt%, the TL signal shows two well defined peaks located at 136 and 253 °C due to gamma radiation, and a third less intense broad peak at ≈ 180 °C (Fig. 6a). We can see that all Eu-doped HAp samples exhibits an intensity peak between 130 and 153 °C, depending of the Eu concentration. Moreover, a second or third maximum peak could appear when the Eu concentration is modified.
For all that stated until now, we can affirm that each one of doped HAp samples shows a different thermoluminescent behavior which is corroborated to the differences of the crystallinity and nanostructural characteristics such as crystalline phases present and crystal sizes among them. Furthermore, a detailed analysis will be performed in future work for understanding the crystalline phase effect on TL signal.
For all Eu-doped HAp samples, the graph in Fig. 6b reveals that the relationship between the TL Intensity (I) and dosage (D) in the range from 1.0 to 100 Gy is linear. Although the intensity of all systems was linearly related to the radiation dosage received, their sensitivities to radiation varied depending of the Eu concentration.
According to the Fig. 6b, the thermoluminescent material with the most sensitivity to gamma radiation was the 0.5 wt% Eu-doped HAp. On the other hand, HAp samples with higher concentration of Eu dopant than 0.5 wt% show a minor sensitivity to gamma radiation. We believe that this is because the Eu in HAp tends to segregate or take part of other chemical compound (EuPO4·H2O). Therefore, the presence of dual phase does not contribute to increase the thermoluminescent signal intensity [47].
The fact that all Eu doped samples obtained in this work showed a thermoluminescent behaviour due to gamma irradiation suggest a possibility to use the HAp: Eu nanosystem as gamma radiation dosimeter. However, more thermoluminescent studies are necessary before to consider as dosimeter, such as, the linearity range test, which show the full range of linear relationship between TL response and dosage (Gy), the reproducibility of the TL measurements, the fading test or the process in which there is an unintentional loss of the information [48], which will be our next objective.
Due to the increase in the usage of the gamma irradiation proceess, there is a continuing need to develop new thermoluminescent materials, which are able to measure gamma radiation fields. They must show major advantages respect to other systmes (like small physical size, easy to transport, more sensitive, reusable with only a gradual change in efficiency, more economical).
4 Conclusion
In this work, we have successfully synthesized pure and Eu-doped hydroxyapatite nanorods by using microwave assisted hydrothermal method. Rod-like structures with different width from 20 to 40 nm and ~ 50 nm of length were observed. The aspect ratio of these structures depends on the Eu added during the synthesis of HAp. All the samples revealed the presence of hexagonal and monoclinic crystalline phases. Furthermore, Rietveld analysis of XRD patterns quantified the proportion of each crystalline phase and the presence of EuPO4·H2O with a minimal proportion of Eu that segregates. It was also found that the increasing of the Eu dopant resulted in decrease in the concentration of hexagonal phase and an increase in the concentration of monoclinic phase. Moreover, the increasing in Eu concentration induced a growth in the crystal size for monoclinic phase, while a decrement in the crystal size of hexagonal phase was observed. Thermoluminescence properties of pure and Eu doped HAp samples were also investigated in this work. Unlike pure HAp, which shows a weak TL signal induced by gamma radiation, Eu-doped HAp demonstrates highly intense TL response and 0.5 wt% Eu doped HAp show the relatively highest TL response. However, the TL curve shape was highly dependent on the Eu concentration. A lower shift in the peak intensity from 153 to 130 °C resulted from an increase in Eu concentration. In addition, a second or third maximum peak appeared at higher temperature for higher doping. This thermoluminescent behavior is based on the crystalline composition and crystal size of each sample, which in turn are dependent on the Eu concertation. Our results show that the dopant induced more trapping centers or recombination centers for obtaining higher TL signal compared to pure HAp. Finally, the fact that doped samples showed a linear relationship between the TL Intensity (I) and dosage (D) in the range from 1 to 100 Gy, which facilitates the development of novel hydroxyapatite materials as radiation sensors specifically for gamma radiation.
References
N. Kantharia, S. Naik, S. Apte, M. Kheur, S. Kheur, B. Kale, J. Dent. Res. Sci. Dev. 1(1), 15–19 (2014)
L.L. Hench, J. Am. Ceram. Soc. 74, 1487–1510 (1991)
N. Roveri, M. Lafisco, Nanotechnol. Sci. Appl. 3, 107–125 (2010)
X.-Y. Zhao, Y.-J. Zhu, J. Zhao, B.-Q. Lu, F. Chen, C. Qi, J. Wu, J. Colloid Interface Sci. 416, 11–18 (2014)
N.F. Mohammad, R. Othman, F. Yee-Yeoh, Rev. Adv. Mater. Sci. 28, 138–147 (2014)
X.-Y. Zhao, Y.-J. Zhu, C. Qi, F. Chen, B.-Q. Lu, J. Zhao, J. Wu, Chem. Asian. J. 8, 1313–1320 (2013)
W. Wei, R. Sun, Z. Jin, J. Cui, Z. Wei, Appl. Surf. Sci. 292, 1020–1029 (2014)
S.L. Iconaru, M. Motelica-Heino, D. Predoi, J. Spectrosc. (2013). https://doi.org/10.1155/2013/284285
V. Rodríguez-Lugo, E. Salinas-Rodríguez, R.A. Vázquez, K. Alemán, A.L. Rivera. RSC Adv. 7, 7631–7639 (2017)
V. Rodríguez Lugo, V.M. Castaño, E. Rubio-Rosas, Mater. Lett. 184, 265–268 (2016)
V. Rodríguez-Lugo, C. Ángeles, A. de la Isla, V.M. Castaño, Int. J. Basic Appl. Sci. 4(4), 395–403 (2015)
V. Rodríguez-Lugo, J. Sánchez Hernández, M.J. Arellano-Jiménez, P.H. Hernández-Tejeda, S. Recillas-Gispert, Microsc. Microanal. 11(6), 516–523 (2005)
V. Rodríguez-Lugo, M. Hernández, C. Angeles-Chavez, Mater. Manuf. Process. 18(6), 903–913 (2003)
V. Rodríguez-Lugo, J.A. Ascencio, C. Angeles-Chavez, A. Camacho-Bragado, V.M. Castaño, Mater. Technol. 16, 97–103 (2001)
X. Zheng, M. Liu, J. Hui, D. Fan, H. Ma, X. Zhang, Y. Wang, Y. Wei, Phys. Chem. Chem. Phys. 17, 20301–20307 (2015)
F. Ziaie, N. Hajiloo, A. Alipour, R. Amraei, S.I. Mehtieva, Radiat. Prot. Dosim. 145(4), 377–384 (2011)
A. Zarinfar, M. Shafaei, F. Ziaie, Proced. Mater. Sci. 11, 293–298 (2015)
R. Alvarez, T. Rivera, J. Guzman, M.C. Piña-Barba, J. Azorin, Appl. Radiat. Isot. 83, 192–195 (2014)
M. Shafaei, F. Ziaie, D. Sardari, M.M. Larijani, Kerntechnik 80(1), 66–69 (2015)
J. Zarate-Medina, K.J. Sandoval-Cedeño, A. Barrera-Villatoro, J. Lemus-Ruiz, T. Rivera-Montalvo, Appl. Radiat. Isot. 100, 50–54 (2015)
C. Woda, C. Bassinet, F. Trompier, E. Bortolin, S.D. Monaca, P. Fattibene, Ann. Ist. Sanita 45(3), 297–306 (2009)
K. Madhukumar, H.K. Varma, M. Komath, T.S. Elias, V. Padmanabhan, M.K. Nair, Bull. Mater. Sci. 30(5), 527–534 (2007)
N. Salah, S.S. Habib, Z.H. Khan, F. Djouider, Radiat. Phys. Chem. 80, 923–928 (2011)
G. Villa-Sánchez, D. Mendoza-Anaya, G. Mondragon-Galicia, R. Pérez-Hernández, P.R. González-Martínez, O.F. Olea-Mejía, J. Radiat. Phys. Chem. 97, 118–125 (2014)
G. Villa-Sánchez, D. Mendoza-Anaya, M.E. Fernández-García, L. Escobar-Alarcón, O.F. Olea Mejía, P.R. González-Martínez, Opt. Mater. 14, 1219–1226 (2014)
J.A. Nieto, AIP Conf. Proc. (2004). https://doi.org/10.1063/1.1811814
S.W. Lowe, S. Bodis, A. McClatchey, L. Remngton, H. Earl Ruley, D.E. Fisher, D.E. Housman, T. Jacks, Science 266(5186), 807–810 (1994)
N.I. Zakariya, M. Kahn, J. Biosci. 2(9), 583–591 (2014)
K.A. da Silva Aquino, ISBN: 978-953-51-0316-5, InTech, (2012). http://www.intechopen.com/books/gammaradiation/sterilization-by-gamma-irradiation
M. Pricaz, A.-C. Utâ., Romanian J. Biophys. 25(2), 143–162 (2015)
J.M.M. Walder, C.O. Calkins, Sci. Agric. Piracícaba 50(2), 157–165 (1993)
V.J. Angadi, A.V. Anupama, R. Kumar, H.M. Somashekarappa, K. Praveena, B. Rudraswamy, B. Sahoo, Ceram. Int. 2, 5933–15939 (2016)
J. Angadi, S. Matteppanavar, R.B. Katti, B. Rudraswamy, K. Praveena, AIP Conf. Proc. (2017). https://doi.org/10.1063/1.4980760
Teodoro Rivera. INTECH open science/open minds, 127–164 (2011). https://doi.org/10.5772/17423. http://www.intechopen.com
S. Katlakunta, S.S. Meena, S. Srinath, M. Bououdinad, R. Sandhya, K. Praveena, Mater. Res. Bull. 63, 58–66 (2015)
K. Sadhana, S.R. Murthy, K. Praveena, Mater. Sci. Semicond. Process. 34, 305–311 (2015)
P. Kuruva, P.R. Matli, B. Mohammad, S. Reddigari, S. Katlakunta, J. Magn. Magn. Mater. 382, 172–178 (2015)
K. Praveena, K. Sadhana, H.-L. Liu, N. Maramu, G. Himanandini, J. Alloys Compd. 681, 499–507 (2016)
K. Sadhana, R. Sandhya, S.R. Murthy, K. Praveena, Mater. Focus 3(4) (2014)
K. Praveena, H.-W. Chen, H.-L. Liu, K. Sadhana, S.R. Murthy, J. Magn. Magn. Mater. 420, 129–142 (2016)
S. Katlakunta, P. Raju, S.S. Meena, S. Srinath, R. Sandhya, P. Kuruva, S.R. Murthy, Phys. B Condens. Matter 448, 323–326 (2014)
A. Verma, R. Dwivedi, R. Prasad, K.S. Bartwal, J. Nanoparticles (2013). https://doi.org/10.1155/2013/737831
Z. Zhang, C.-C. Wang, R. Zakaria, J.Y. Ying, J. Phys. Chem. B 52, 10871–10878 (1998)
G. Ma, X.Y. Liu, Cryst. Growth Design 9(7), 2991–2994 (2009)
H. Suda, M. Yashima, M. Kakihana, M. Yoshimura, J. Phys. Chem. 99, 6752–6754 (1995)
S. Kuśnieruk, J. Wojnarowicz, A. Chodara, T. Chudoba, S. Gierlotka, W. Lojkowski, Beilstein J. Nanotechnol. 7, 1586–1601 (2016). https://doi.org/10.3762/bjnano.7.153
M. Shafaei, F. Ziaie, D. Sardaria, M.M. Larijani. Luminescence 31, 223–228 (2015)
P.R. González, D. Mendoza-Anaya, L. Escobar-Alarcón, J. Luminesc. 195 (2017). https://doi.org/10.1016/j.jlumin.2017.11.050
Acknowledgements
This work was financially supported by the project PROMEP– UAEH– PTC–6669. Authors would like to thank to the Electron Microscopy and X-Ray Diffraction Laboratories at the ININ, CONACyT SENER-HIDROCARBUROS postdoctoral project and the CONACyT project INFR-2015-251767.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mendoza-Anaya, D., Flores-Díaz, E., Mondragón-Galicia, G. et al. The role of Eu on the thermoluminescence induced by gamma radiation in nano hydroxyapatite. J Mater Sci: Mater Electron 29, 15579–15586 (2018). https://doi.org/10.1007/s10854-018-9147-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9147-4