Abstract
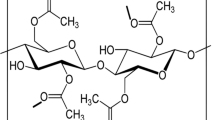
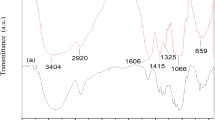
In this present work, the solution casting technique was utilized to develop the proton conducting solid biopolymer electrolyte by the complex formation of cellulose acetate (CA) with the ammonium thiocyanate (NH4SCN) salt. The crystalline nature and complex formation of CA with different concentrations of NH4SCN were investigated using X-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectroscopic techniques. The XRD analysis revealed that the amorphous natures of the CA complex were increased with increase of NH4SCN salt concentration, which leads to the higher ionic conductivity. The FTIR analysis confirmed the complex formation between CA and salt matrix. Differential scanning calorimetry (DSC) was used to predict the glass transition temperature (Tg) values, which reveals that the Tg value increase with respect to the increase of NH4SCN concentration. The electrical conductivity was measured using AC impedance analyzer, which showed that the magnitude of ionic conductivity increases with an increase in salt concentration up to 50CA:50NH4SCN. The 50CA:50NH4SCN has maximum ionic conductivity value of 3.31 × 10−3 S cm−1. Transference number measurement was carried out to investigate the nature of the charge transport species in the polymer electrolyte. The proton battery was constructed with the highest conducting polymer electrolyte 50CA:50NH4SCN and its open circuit voltage with load were studied. Hence, the present investigation paves the way for the development of fuel cell and primary proton battery applications.












Similar content being viewed by others
References
A. Karmakar, A. Ghosh, Dielectric permittivity and electric modulus of polyethyleneoxide (PEO)-LiClO4 composite electrolyte. Curr. Appl. Phys. 12(2), 539–543 (2012)
W.H. Meyer, Polymer electrolytes for lithium-ion batteries. Adv. Mater. 10(6), 439–448 (1998)
A.S. Ahmad Khiar, A.K. Arof, Conductivity studies of starch-based polymer electrolytes. Ionics 16, 123–129 (2010)
K. Pradeep, Varshney, Shikha Gupta, natural polymer-based electrolytes for electrochemical devices: a review. Ionics 17, 479–483 (2011)
L. Ponez, F.C. Sentanin, S.R. Majid, A.K. Arof, A. Pawlicka, Ion-conducting electrolytes based on gelatin and containing LiI/I2 for electrochromic devices. Mol. Cryst. Liq. Cryst. 554, 239–251 (2012)
S.B. Aziz, Z.H.Z. Abidin, A.K. Arof, Effect of silver nanoparticles on the dc conductivity in Chitosan silvertriflate polymer electrolyte. Phys. B 405, 4429–4433 (2010)
N.A. Nik Aziz, N.K. Idris, M.I.N. Isa, Proton conducting polymer electrolyte methylcellulose doped ammonium fluoride: conductivity and ionic transport study. Int. J. Phys. Sci. 5(6), 748–752 (2010)
A. Daniel Cerqueira, J.M. Artur Valente, R. Guimes Filho, D. Hugh Burrows, Synthesis and properties of polyaniline-cellulose acetate blends: the use of sugarcane bagasse waste and the effect of the substitution degree. Carbohydr. Polym. 78, 402–408 (2009)
S. Ramesh, R. Shanthi, Ezra Morris, characterization of conducting cellulose acetate based polymer electrolytes doped with “green” ionic mixture. Carbohydr. Polym. 9, 14–21 (2013)
S. Ramesh, R. Shanthi, Ezra Morris, plactizing effect of 1-allyl-3-methylimidazolium chloride in cellulose acetate based polymer electrolytes. Carbohydr. Polym. 87, 2624–2629 (2012)
N.A. Johari, T.I.T. Kudin, A.M.M. Ali, T. Winie, M.Z.A. Yahya, Studies on cellulose acetate-based gel polymer electrolytes for proton batteries. Mater. Res. Innov. 13(3), 232–234 (2009)
N.A. Johari, T.I.T. Kudin, A.M.M. Ali, M.Z.A. Yahya, Electrochemical studies of composite cellulose acetate-based polymer gel electrolytes for proton. Proc. Natl. Acad. Sci. Sect. A Phys. Sci. 82(1), 49–52 (2009)
G.M. Wu, S.J. Lin, C.C. Yang, in Fuel Cell Research Trends, ed. by L.O. Vasquez (Nova Science Publishers, Inc., Newyork, 2007), p. 448
S.Z.Z. Abidin, A.M.M. Ali, O.H. Hassan, M.Z.A. Yahya, Electrochemical studies on cellulose acetate-LiBOB polymer gel electrolytes. Int. J. Electrochem. Sci. 8, 7320–7326 (2013)
M. Selvakumar, D. Krishna Bhat, LiClO 4 Doped Cellulose Acetate as Biodegradable Polymer Electrolyte for Supercapacitors (Wiley, Hoboken, 2008). doi:10.1002/app.28671
S. Chandra, S.A. Hashmi, G. Prasad, Studies on ammonium perchlorate doped polyethyleneoxide polymer electrolyte. Solid State Ion. 40–41, 651–654 (1990)
M. Kumar, S. Sekhon, Role of plasticizer’s dielectric constant on conductivity modification of PEO-NH4F polymer electrolytes. Eur. Polym. 38, 1297–1304 (2002)
F.M. Gray, Solid Polymer Electrolytes (VCH Publishers Inc, New York, 1991)
S.A. Hashmi, A. Kumar, K.K. Maurya, S. Chandra, Proton-conducting polymer electrolyte. I. The polyethylene oxide + NH4ClO4 system. J. Phys. D Appl. Phys. 23(10), 1307 (1993)
N.A. Aziza, S.R. Majida, R. Yahyaa, A.K. Arof, Conductivity, structure, and thermal properties of chitosan-based polymer electrolytes with nanofillers. Wiley Online Lib. (2009). doi:10.1002/pat.1619
M.F. Shukur, Y.M. Yusof, S.M.M. Zawawi, H.A. Illias, M.F.Z. Kadir, Conductivity and transport studies of plasticized Chitosan-based proton conducting biopolymer electrolytes. Phys. Scr. 57, 014050 (2013)
M.P. Aji, Masturi, S. Bijaksana, Khairurrijal, M. Abdullah, A general formula for ion concentration dependent electrical conductivities in polymer electrolytes. Am. J. Appl. Sci. 9(6), 946–954 (2012)
R. Baskaran, S. Selvasekarapandian, N. Kuwata, J. Kawamura, T. Hattori, Conductivity and thermal studies of blend polymer electrolytes based on PVAc–PMMA. Solid State Ion. 177(26), 2679–2682 (2006)
R.M. Hodge, G.H. Edward, G.P. Simon, Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 37, 1371–1376 (1996)
M.F.Z. Kadir, S.R. Majid, A.K. Arof, Plasticized chitosan–PVA blend polymer electrolyte based proton battery. Electrochim. Acta 55, 1475–1482 (2010)
M. Ali, Influence of glycol additives on the structure and performance of cellulose acetate/zinc oxide blend electrolytes. Desalination 270, 98–104 (2011)
G. Hirankumar, S. Selvasekarapandian, M.S. Bhuvaneswari, R. Baskaran, M. Vijayakumar, AC impedance studies on proton conducting polymer electrolyte complexes (PVA + CH3 COONH4). Ionics 10, 135–138 (2004)
M. Xu, E.M. Eyring, S. Petrueei, Molecular dynamics and infrared spectra of NaSCN dissolved in the solvent macrocycle 15-crown-5 and polyethylene oxide dimethyl ether-250. J. Phys. Chem. 99(40), 14589–14596 (1995)
H. Zhang, X. Xuan, J. Wang, H. Wamg, FT-IR investigation of ion association in PEO–MSCN (M = Na, K) polymer electrolytes. Solid State Ion. 164, 73–79 (2003)
A. Pottier, The Hydrogen Bond and Chemical Parameters Favoring Proton Mobility in Solid, in Proton Conductors: Solid, Electrolytes and Gel Materials and Devices (Cambridge University Press, Cambridge, 1992)
T. Norby, Solid State Ion. 125, 1–11 (1999)
B.H. Stuar, Infrared Spectroscopy: Fundamentals and Applications (Wiley, Colorado, 2004)
H. Nithya, Ph.D thesis entitled Characterization of polymer electrolyte poly(Epichlorohydrin–ethyleneoxide): LiClO4 (2011)
C.A. Angell, K. Xu, S.S. Zhang, N. Videa, Variations on the salt-polymer electrolyte theme for flexible solid electrolytes. Solid State Ion. 86–88, 17–28 (1996)
W.A. Gazotti, M.A.S. Spinacé, E.M. Girotto, M.A. De Paoli, Polymer electrolytes based on ethylene oxide–epichlorohydrin copolymers. Solid State Ion. 130, 281–291 (2000)
G.G. Silva, N.H.T. Lemes, C.N. Polo da Fonseca, M.A. De Paoli, Solid state polymeric electrolytes based on poly (epichlorohydrin). Solid State Ion. 93, 105–116 (1997)
J.R. Macdonald (ed.), Impedance Spectroscopy (Wiley, New York, 1987)
B.A. Boukamp, Solid State Ion. 20, 301 (1986)
B.A. Boukamp, Solid State Ion. 18&19, 136 (1986)
H. Nithya, S. Selvasekarapandian, P. ChristopherSelvin, D. ArunKumar, M. Hemaa, D. Prakash, Characterization of nanocomposite polymer electrolyte based on P(ECH-EO). Phys. B 406, 3367–3373 (2011)
S.H. Kim, J.Y. Kim, H.S. Kim, H.N. Cho, Ionic conductivity of polymer electrolytes based on phosphate and polyether copolymers. Solid State Ion. 116, 63–71 (1999)
C. Kim, G. Lee, K. Leo, K.S. Ryu, S.H. Chang, Polymer electrolytes prepared by polymerizing mixtures of polymerizable PEO-oligomers, copolymer of PVDC and poly(acrylonitrile), and lithium triflate. Solid State Ion. 123, 251–257 (1999)
A.K. Jonscher, The universal dielectric response. Nature 267, 673–679 (1977)
N. Rajeswari, C. Sanjeeviraja, J. Kawamura, S. Asath Bahadur, A study on polymer blend electrolyte based on PVA/PVP with proton salt. Polym. Bull. (2014). doi:10.1007/s00289-014-1111-8
J.R. Mac Callum, C.A. Vincent, low frequency dielectric properties of polyether electrolytes. Elsevier Appl. Sci. 43–60 (1989)
R. Mishra, K.J. Rao, Electrical conductivity studies of poly(ethyleneoxide)-poly(vinylalcohol) blends. Solid State Ion. 106, 113–127 (1998)
R. Baskaran, S. Selvasekarapandian, N. Kuwata, J. Kawamura, T. Hattori, Ac impedance, DSC and FT-IR investigations on (x) PVAc–(1_x) PVdF blends with LiClO4. Mater. Chem. Phys. 98, 55–61 (2006)
K. Adachi, O. Urakawa, Dielectric study of concentration fluctuations in concentrated polymer solutions. J. Non-Cryst. Solids 307–310, 667 (2002)
S. Ramesh, A.K. Arof, Ionic conductivity studies of plasticized poly(vinyl chloride) polymer electrolytes. Mater. Sci. Eng. B 85, 11–15 (2001)
J.B. Wagner, C.J. Wagner, Electrical conductivity measurements on cuprous halides. Chem. Rev. 26, 1597 (1957)
T. Winnie, A.K. Arof, Transport properties of hexanoyl chitosan based gel electrolyte. Ionics 12, 149–152 (2006)
S. Chandra (ed.), Superionic Solids—Principles and Applications, North Holland, Amsterdam (1981)
K. Singh, R.U. Tiwari, V.K. Deshpande, Performance of a solid-state battery with a proton-conducting electrolyte. J. Power Sour. 1, 65–71 (1993)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monisha, S., Selvasekarapandian, S., Mathavan, T. et al. Preparation and characterization of biopolymer electrolyte based on cellulose acetate for potential applications in energy storage devices. J Mater Sci: Mater Electron 27, 9314–9324 (2016). https://doi.org/10.1007/s10854-016-4971-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4971-x