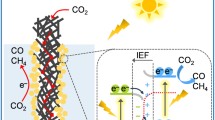
Abstract
CO2 photoconversion to solar fuels requires materials with a high affinity to the acidic CO2, and MgO and Mg(OH)2 films represent good candidates due to their basic sites are highly active for CO2 capture in a wide interval of temperatures. However, the deposition of MgO and Mg(OH)2 as thin film is difficult to obtain by traditional methods. As an alternative, in this work, the successive ionic layer adsorption and reaction (SILAR) method is proposed to obtain MgO/Mg(OH)2 mixtures over glass substrates at significantly lower temperatures (200–400 °C). The films were tested as photocatalysts in the CO2 photoconversion to solar fuels (HCOOH and CH3OH) under UV–visible-NIR irradiation. The as-prepared films exhibited the hexagonal structure of the Mg(OH)2 phase. As the temperature increased, XRD and XPS analysis confirmed the presence of orthorhombic MgO, while the morphology remains with similar grains with an estimated size of 1 µm. The annealing temperatures change the chemical species (Mg–O, Mg–OH, and Mg–CO3) on the films affected their photocatalytic activity. The films exhibited high affinity for CO2 due to the presence of defects (F and F+ centers) in both phases. The photocatalytic behavior was directly related to OH− species present in each sample. According to the results, it seems that fewer hydroxides and defects on the films favored higher efficiencies for the CO2 photoconversion. In addition, the films were exposed to accelerated weathering tests to evaluate their efficiency for more extended periods. The results indicated that the aged films still have activity for CO2 photoconversion.
Graphical abstract













Similar content being viewed by others
References
Güneya H, İskenderoğlu D (2018) Synthesis of MgO thin films grown by SILAR technique. Ceram Int 44(7788):7793
Feng H, Shiyong Wu, Huang S, Youqing Wu, Gao J (2015) Regenerable magnesium-based sorbent for high-pressure and moderate-temperature CO2 capture: physicochemical structures and capture performances. Fuel 159(559):569
Kato K, Omoto H, Takamatsu A, Tomioka T (2011) Crystal growth of MgO thin films deposited on ZnO underlayers by magnetron sputtering. J Cryst Growth 333(59):65
Cáceres D, Vergara I, González R (2003) Microstructural characterization of MgO thin films grown by radio-frequency sputtering. Target and substrate-temperature effect. J Appl Phys 93(4300):4305
Li M, Wang X, Li H, Di H, Xiaoguo Wu, Fang C, Yan B (2013) Preparation of photoluminescent single crystalline MgO nanobelts by DC arc plasma jet CVD. Appl Surf Sci 274(188):194
Suárez-Campos G, Cabrera-German D, García-Valenzuela JA, Cota-Leal M, Fuentes-Ríos JL, Martínez-Gil M, Hu H, Sotelo-Lerma M (2019) Controlled synthesis of Mg(OH)2 thin films by chemical solution deposition and their thermal transformation to MgO thin films. Ceram Int 45(10356):10363
Garza-Hernández R, Lugo-Loredo S, Aguirre-Tostado FS (2020) The role of copper during the growth of stoichiometric Cu2ZnSnS4 by successive ionic layer adsorption and reaction method. Ceram Int 46(5185):5192
Arora S (2020) ZnO/MgO/ITO structured thin film transistor for ultraviolet photo detector application. Mater Today Proc 30(150):152
Khaleel WA, Sadeq SA, Alani IAM, Ahmed MHM (2019) Magnesium oxide (MgO) thin film as saturable absorber for passively mode locked erbium-doped fiber laser. Opt Laser Technol 115(331):336
Taşer A, Güldüre ME, Güney H (2021) Fe doping effects in MgO thin films grown with SILAR technique. Mater Chem Phys 272:124993
Taşer A, Güldüre ME, Güney H (2021) Tuning PL emission energy and bandgap with Ni dopant of MgO thin films. Ceram Int 47(15792):15800
Alfaro Cruz MR, Garza-Hernández R, Horley PP, Mata-Ramírez J, Martínez-G E, Aguirre-Tostado FS (2018) Low temperature ZnO films grown by successive ionic layer adsorption and reaction method. Thin Solid Films 663(49):55
Nicolau YF (1985) Solution deposition of thin solid compound films by a successive ionic layer absorption and reaction process. Appl Surf Sci 22(1061):1074
Zheng Y, Cao L, Xing G, Bai Z, Huang J, Zhang Z (2019) Microscale flower-like magnesium oxide for highly efficient photocatalytic degradation of organic dyes in aqueous solution. RSC Adv 9:7338
Mageshwari K, Sathyamoorthy R (2012) Studies on photocatalytic performance of MgO nanoparticles prepared by wet chemical method. Trans Indian Inst Met 65(49):55
Akbari S, Moussavi G, Giannakis S (2021) Efficient photocatalytic degradation of ciprofloxacin under UVA-LED, using S, N-doped MgO nanoparticles: synthesis, parametrization and mechanistic interpretation. J Mol Liq 324:114831
Chang X, Wang T, Gong J (2016) CO2 photo-reduction: insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ Sci 9(2177):2196
Mahmodi G, Sharifnia S, Madani M, Vatanpour V (2013) Photoreduction of carbon dioxide in the presence of H2, H2O and CH4 over TiO2 and ZnO photocatalysts. Sol Energy 97(186):194
Habisreutinger SN, Schmidt-Mende L, Stolarczyk JK (2013) Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Chem Int Ed 52(7372):7408
Yang C, Tan Q, Li Q, Zhou J, Fan J, Li B, Sun J, Lv K (2020) 2D/2D Ti3C2 Xene/g-C3N4 nanosheets heterojunction for high efficient CO2 reduction photocatalyst: dual effects of urea. Appl Catal 268:118738
Li J, Li K, Tan Q, Li Q, Fan J, Chao Wu, Lv K (2022) Facile preparation of highly active CO2 reduction (001)TiO2/Ti3C2Tx photocatalyst from Ti3AlC2 with less fluorine. Catalysts 12:785
Xiong X, Zhao Y, Shi R, Yin W, Zhao Y, Waterhouse GIN, Zhang T (2020) Selective photocatalytic CO2 reduction over Zn-based layered double hydroxides containing tri or tetravalent metals. Sci Bull 65:987–994
Zhao Y, Chen G, Bian T, Zhou C, Waterhouse GIN, Li-Zhu Wu, Tung C-H, Smith LJ, O’Hare D, Zhang T (2015) Defect-rich ultrathin ZnAl-layered double hydroxide nanosheets for efficient photoreduction of CO2 to CO with water. Adv Mater 27:7824–7831
Shuqi Wu, Wang J, Li Q, Huang Z, Rao Z, Zhou Y (2021) Bi/BiOCl nanosheets enriched with oxygen vacancies to enhance photocatalytic CO2 reduction. Trans Tianjin Univ 27:155–164
An W, Tian L, Jinshan Hu, Liu Li, Cui W, Liang Y (2020) Efficient degradation of organic pollutants by catalytic ozonation and photocatalysis synergy system using double-functional MgO/g-C3N4 catalyst. Appl Surf Sci 534:147518
ASTM International. Standard Guide to Charge Control and Charge Referencing Techniques in X-Ray Photoelectron Spectroscopy. Designation: E1523-15. https://www.astm.org/
Wang L, Wei B, Dong P, Miao Q, Liu Z, Fubiao Xu, Jingjie Wu, Lou J, Vajtai R, Fei W (2016) Large-scale synthesis of few-layer graphene from magnesium and different carbon sources and its application in dye-sensitized solar cells. Mater Des 92(462):470
NIST X-ray photoelectron spectroscopy database. NIST Standard Reference Database 20, Version 4.1
Chen M, Chen Y, Zhang W, Zhao S, Wang J, Mao J, Li W, Zhao Y, Huang N, Wan G (2016) Controlling the corrosion rate and behavior of biodegradable magnesium by a surface immobilized ultrathin 1-hydroxyethylidene-1,1-diphosphonic acid (HEDP) film. RSC Adv 6(15247):15259
Luévano-Hipólito E, Torres Martínez LM (2018) Mg(OH)2 films prepared by ink-jet printing and their photocatalytic activity in CO2 Reduction and H2O Conversion. Top Catal 61(1574):1584
Feliu S, Maffiotte C, Galván JC, Pardo A, Merino MC, Arrabal R (2011) The application of X-ray photoelectron spectroscopy in understanding corrosion mechanisms of magnesium and Mg–Al alloys. Open Surf Sci J 3(1):14
Zhang H, Cao T, Cheng Yi (2014) Synthesis of nanostructured MgO powders with photoluminescence by plasma-intensified pyrohydrolysis process of bischofite from brine. Green Process Synth 3(215):222
Latha Kumari WZ, Li CH, Vannoy RM, Leblanc DZW (2009) Synthesis, characterization and optical properties of Mg(OH)2 micro-/nanostructure and its conversion to MgO. Ceram Int 35(3355):3364
Yousefi S, Ghasemi B, Nikolova MP (2021) Opto-structural characterization of Mg(OH)2 and MgO nanostructures synthesized through a template-free sonochemical method. Appl Phys A 127:549
Jacques J, Geoff T (2015) Defects at oxide surfaces. Springer, Cham
Wang K, Tao Wu, Congcong Wu, Rammohan Sriramdas Xu, Huang KW, Jiang Y, Liu H, Yan Y, Yang D, Ye T, Liu C, Xiaowen Hu, Jiang X, Priya S (2020) Nature of terrace edge states (TES) in lower dimensional halide perovskite. J Mater Chem A 8(7659):7670
Ki Young Kim (2010) Recent optical and photonic technologies. INTECH, Croatia, pp 450
Nemade KR, Waghuley SA (2014) Synthesis of MgO nanoparticles by solvent mixed spray pyrolysis technique for optical investigation. Int J Met. https://doi.org/10.1155/2014/389416
Habisreutinger SN, Schmidt-Mende L, Stolarczyk JK (2013) Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew Chem Int Ed 52(7372):7408
Luévano-Hipólito E, Torres-Martínez LM (2022) CO2 photoreduction with H2O to C1 and C2 products over perovskite films of alkaline niobates ANbO3 (A = Li, Na, K). Fuel 320:123934
Wang H, Cheng S, Cai X, Cheng L, Zhou R, Hou T, Li Y (2022) Photocatalytic CO2 reduction to HCOOH over core-shell Cu@Cu2O catalysts. Catal Commun 162:106372
Henglein A, Gutiérrez M, Fischer C-H (1984) Photochemistry of colloidal metal sulfides 6. Kinetics of interfacial reactions at ZnS-particles. Ber Bunsenges Phys Chem 88(2):175
Zhao X, Fan Y, Zhang W, Zhang X, Han D, Niu L, Ivaska A (2020) Nanoengineering construction of Cu2O nanowire arrays encapsulated with g-C3N4 as 3D spatial reticulation all-solid-state direct Zscheme photocatalysts for photocatalytic reduction of carbon dioxide. ACS Catal 10(6367):6376
Song H, Meng X, Wang S, Zhou W, Song S, Kako T, Ye J (2020) Selective photo-oxidation of methane to methanol with oxygen over dual-cocatalyst-modified titanium dioxide. ACS Catal 10(14318):14326
Yang C, Li Q, Xi Y, Lv K, Li M (2019) Enhanced visible-light photocatalytic CO2 reduction performance of Znln2S4 microspheres by using CeO2 as cocatalyst. Appl Surf Sci 464(388):395
Li K, Zhang S, Li Y, Fan J, Lv K (2021) MXenes as noble-metal-alternative co-catalysts in photocatalysis. Chin J Catal 42(1):14
Li Y, Ren Z, Gu M, Duan Y, Zhang W, Lv K (2022) Synergistic effect of interstitial C doping and oxygen vacancies on the photoreactivity of TiO2 nanofibers towards CO2 reduction. Appl Catal B 317:121773
Acknowledgements
The authors want to thank CONACYT for financial support for this research through the following projects: FC-2016-1725, Paradigmas y Fronteras de la Ciencia 320379, and Cátedras-CONACYT 363, and 1060. In addition, thanks are extended to the UANL for its financial support through the projects: PAYCIT: CE1771-21, IT1766-21, and 277-CE-2022. We also thank Dr. Alonso Concha Balderrama and M.C. Luis Gerardo Silva Vidaurri from CIMAV-Monterrey for his technical support in the XRD and XPS measurements.
Funding
Authors declare they have no financial interests. This work was supported by the following CONACYT projects: FC-2016-1725, Paradigmas y Fronteras de la Ciencia 320379, and Cátedras-CONACYT 363, and 1060. Also, UANL was supported part of the work with the projects: PAYCIT: CE1771-21, IT1766-21, 277-CE-2022.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design of this work. MRAC and EL-H contributed to the conceptualization, methodology, investigation. MRAC, EL-H, and RG-H contributed to the writing—original draft, writing—review, and editing. While LMT-M contributed to the conceptualization, project administration, founding acquisition, resources, writing—review, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Handling Editor: David Cann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cruz, M.R.A., Luévano-Hipólito, E., Garza-Hernández, R. et al. MgO and Mg(OH)2 thin films prepared by the SILAR method and their CO2 photocatalytic performance. J Mater Sci 57, 18739–18753 (2022). https://doi.org/10.1007/s10853-022-07837-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07837-x