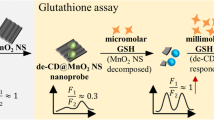
Abstract
The utilization of nanomaterial-based probes in detecting glutathione (GSH) and cell imaging has aroused extensive attention owing to the excellent properties of nanoprobes. Herein, we have synthesized manganese dioxide-S, O co-doped graphitic carbon nitride quantum dots (MnO2-S, O-CNQDs) nanocomposite by in situ synthesis of MnO2 nanosheets in S,O-CNQDs dispersion solution. It was found that GSH could specifically bind to MnO2-S, O-CNQDs so that the fluorescence of S, O-CNQDs could be recovered. As such, a “turn-on” MnO2-S, O-CNQDs nanoprobe can be fabricated and applied to rapidly determine trace amounts of GSH. Under the optimal conditions, MnO2-S, O-CNQDs shows sensitive response to GSH in the range 10–270 μM with a detection limit of 0.307 μM (S/N = 3). The developed MnO2-S, O-CNQDs probe has demonstrated great potential to detection of GSH in biological samples and glutathione injections. What is more, MTT assay indicates that MnO2-S, O-CNQDs has low biotoxicity. The non-fluorescence MnO2-S, O-CNQDs reacts with GSH to recover the fluorescence of S, O-CNQDs in HepG2 cells. Thus, the “turn-on” fluorescence change of MnO2-S, O-CNQDs offers a potentially useful tool to monitor GSH of cancer cells.
Graphical Abstract











Similar content being viewed by others
References
Kong RM, Ma L, Han X, Ma C, Qu F, Xia L (2020) Hg2+-mediated stabilization of G-triplex based molecular beacon for label-free fluorescence detection of Hg2+, reduced glutathione, and glutathione reductase activity. Spectrochim Acta A Mol Biomol Spectrosc 228:117855. https://doi.org/10.1016/j.saa.2019.117855
Lv H, Zhen C, Liu J, Yang P, Hu L, Shang P (2019) Unraveling the potential role of glutathione in multiple forms of cell death in cancer therapy. Oxid Med Cell Longev 2019:3150145. https://doi.org/10.1155/2019/3150145
Smeyne M, Smeyne RJ (2013) Glutathione metabolism and Parkinson’s disease. Free Radic Biol Med 62:13–25. https://doi.org/10.1016/j.freeradbiomed.2013.05.001
Lu SC, Mato JM, Espinosa-Diez C, Lamas S (2016) MicroRNA-mediated regulation of glutathione and methionine metabolism and its relevance for liver disease. Free Radic Biol Med 100:66–72. https://doi.org/10.1016/j.freeradbiomed.2016.03.021
Pocernich CB, Butterfield DA (2012) Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochim Biophys Acta 5:625–630. https://doi.org/10.1016/j.bbadis.2011.10.003
Perricone C, De Carolis C, Perricone R (2009) Glutathione: a key player in autoimmunity. Autoimmun Rev 8(8):697–701. https://doi.org/10.1016/j.autrev.2009.02.020
Iwasaki Y, Saito Y, Nakano Y, Mochizuki K, Sakata O, Ito R, Saito K, Nakazawa H (2009) Chromatographic and mass spectrometric analysis of glutathione in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci 877(28):309–317. https://doi.org/10.1016/j.jchromb.2009.07.001
Hanko M, Svorc L, Plankova A, Mikus P (2019) Overview and recent advances in electrochemical sensing of glutathione: a review. Anal Chim Acta 1062:1–27. https://doi.org/10.1016/j.aca.2019.02.052
Saha A, Jana NR (2013) Detection of cellular glutathione and oxidized glutathione using magnetic-plasmonic nanocomposite-based “turn-off” surface enhanced Raman scattering. Anal Chem 85(19):9221–9228. https://doi.org/10.1021/ac4019457
Wawegama NK, Browning GF, Kanci A, Marenda MS, Markham PF (2014) Development of a recombinant protein-based enzyme-linked immunosorbent assay for diagnosis of Mycoplasma bovis infection in cattle. Clin Vaccine Immunol 21(2):196–202. https://doi.org/10.1128/cvi.00670-13
Chen JA, Zhang ZY, Gao J, Tan JH, Gu XF (2019) Design of fluorescent probes with optimized responsiveness and selectivity to GSH. Tetrahedron Lett 60(18):1226–1230. https://doi.org/10.1016/j.tetlet.2019.03.051
Li Y, Jiang L, Zou Y, Song Z, Jin S (2021) Highly reproducible SERS sensor based on self-assembled Au nanocubic monolayer film for sensitive and quantitative detection of glutathione. Appl Surf Sci 540:148381. https://doi.org/10.1016/j.apsusc.2020.148381
Zhang L, Ling B, Wang L, Chen H (2017) A near-infrared luminescent Mn2+-doped NaYF4:Yb, Tm/Fe3+ upconversion nanoparticles redox reaction system for the detection of GSH/Cys/AA. Talanta 172:95–101. https://doi.org/10.1016/j.talanta.2017.05.031
Zhang Y, Zhang W, Chen K, Yang Q, Hu N, Suo Y, Wang J (2018) Highly sensitive and selective colorimetric detection of glutathione via enhanced Fenton-like reaction of magnetic metal organic framework. Sens Actuators B Chem 262:95–101. https://doi.org/10.1016/j.snb.2018.01.221
Chu S, Wang H, Du Y, Yang F, Yang L, Jiang C (2020) Portable smartphone platform integrated with a nanoprobe-based fluorescent paper strip: visual monitoring of glutathione in human serum for health prognosis. ACS Sustain Chem Eng 8(22):8175–8183. https://doi.org/10.1021/acssuschemeng.0c00690
Pan J, Zheng Z, Yang J, Wu Y, Lu F, Chen Y, Gao W (2017) A novel and sensitive fluorescence sensor for glutathione detection by controlling the surface passivation degree of carbon quantum dots. Talanta 166:1–7. https://doi.org/10.1016/j.talanta.2017.01.033
He F, Wang Z, Li Y, Peng S, Liu B (2020) The nonmetal modulation of composition and morphology of g-C3N4-based photocatalysts. Appl Catal B 269:118828. https://doi.org/10.1016/j.apcatb.2020.118828
Wang L, Tong Y, Feng J, Hou J, Li J, Hou X, Liang J (2019) g-C3-N4based films: a rising star for photoelectrochemical water splitting. Sustain Mater Technol 19:e00089. https://doi.org/10.1016/j.susmat.2018.e00089
Bansod B, Kumar T, Thakur R, Rana S, Singh I (2017) A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens Bioelectron 94:443–455. https://doi.org/10.1016/j.bios.2017.03.031
Zhang X, Xie X, Wang H, Zhang J, Pan B, Xie Y (2012) Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J Am Chem Soc 135(1):18–21. https://doi.org/10.1021/ja308249k
Habibi Jouybari M, Hosseini S, Mahboobnia K, Boloursaz LA, Moradi M, Irani M (2019) Simultaneous controlled release of 5-FU, DOX and PTX from chitosan/PLA/5-FU/g-C3N4-DOX/g-C3N4-PTX triaxial nanofibers for breast cancer treatment in vitro. Colloids Surf B Biointerfaces 179:495–504. https://doi.org/10.1016/j.colsurfb.2019.04.026
Taheri H, Unal MA, Sevim M, Gurcan C, Ekim O, Ceylan A, Syrgiannis Z, Christoforidis KC et al (2020) Photocatalytically active graphitic carbon nitride as an effective and safe 2D material for in vitro and in vivo photodynamic therapy. Small 16(10):e1904619. https://doi.org/10.1002/smll.201904619
Zhao Z, Zheng H, Wang Y, Cai X, Mao L, Zhang J (2019) Hydrogen atom etching induced large-size ultrathin g-C3N4 nanosheets for enhanced photoluminescence. J Lumin 206:660–665. https://doi.org/10.1016/j.jlumin.2018.10.080
Naidu PP, Raghavendra G, Ojha S, Paplal B (2019) Effect of g-C3N4 nanofiller as filler on mechanical properties of multidirectional glass fiber epoxy hybrid composites. J Appl Polym Sci 137(9):48413. https://doi.org/10.1002/app.48413
Yang J, Liang Y, Li K, Yang G, Wang K, Xu R, Xie X (2019) Cyano and potassium-rich g-C3N4 hollow tubes for efficient visible-light-driven hydrogen evolution. Catal Sci Technol 9(13):3342–3346. https://doi.org/10.1039/c9cy00925f
Rong M, Cai Z, Xie L, Lin C, Song X, Luo F, Wang Y, Chen X (2016) Study on the ultrahigh quantum yield of fluorescent P, O-g-C3N4 nanodots and its application in cell imaging. Chemistry 22(27):9387–9395. https://doi.org/10.1002/chem.201601065
Yan X, Song Y, Zhu C, Li H, Du D, Su X, Lin Y (2018) MnO2 nanosheet-carbon dots sensing platform for sensitive detection of organophosphorus pesticides. Anal Chem 90(4):2618–2624. https://doi.org/10.1021/acs.analchem.7b04193
Garg D, Mehta A, Mishra A, Basu S (2018) A sensitive turn on fluorescent probe for detection of biothiols using MnO2@carbon dots nanocomposites. Spectrochim Acta A Mol Biomol Spectrosc 192:411–419. https://doi.org/10.1016/j.saa.2017.11.041
Yang J, Huang Z, Hu Y, Ge J, Li J, Li Z (2018) A facile fluorescence assay for rapid and sensitive detection of uric acid based on carbon dots and MnO2 nanosheets. New J Chem 42(18):15121–15126. https://doi.org/10.1039/c8nj02607f
Xue L, Guo R, Huang F, Qi W, Liu Y, Cai G, Lin J (2020) An impedance biosensor based on magnetic nanobead net and MnO2 nanoflowers for rapid and sensitive detection of foodborne bacteria. Biosens Bioelectron 173:112800. https://doi.org/10.1016/j.bios.2020.112800
Yuan J, Cen Y, Kong XJ, Wu S, Liu CL, Yu RQ, Chu X (2015) MnO2-nanosheet-modified upconversion nanosystem for sensitive turn-on fluorescence detection of H2O2 and glucose in blood. ACS Appl Mater Interfaces 7(19):10548–10555. https://doi.org/10.1021/acsami.5b02188
Li Q, Xia Y, Wan X, Yang S, Cai Z, Ye Y, Li G (2020) Morphology-dependent MnO2/nitrogen-doped graphene nanocomposites for simultaneous detection of trace dopamine and uric acid. Mater Sci Eng C Mater Biol Appl 109:110615. https://doi.org/10.1016/j.msec.2019.110615
Zhou J, Yang Y, Zhang CY (2013) A low-temperature solid-phase method to synthesize highly fluorescent carbon nitride dots with tunable emission. Chem Commun 49(77):8605–8607. https://doi.org/10.1039/c3cc42266f
Wang H, Lu Q, Li M, Li H, Liu Y, Li H, Zhang Y, Yao S (2018) Electrochemically prepared oxygen and sulfur co-doped graphitic carbon nitride quantum dots for fluorescence determination of copper and silver ions and biothiols. Anal Chim Acta 1027:121–129. https://doi.org/10.1016/j.aca.2018.03.063
Lu YC, Chen J, Wang AJ, Bao N, Feng JJ, Wang W, Shao L (2015) Facile synthesis of oxygen and sulfur co-doped graphitic carbon nitride fluorescent quantum dots and their application for mercury(ii) detection and bioimaging. J Mater Chem C 3(1):73–78. https://doi.org/10.1039/c4tc02111h
Wu M, Hou P, Dong L, Cai L, Chen Z, Zhao M, Li J (2019) Manganese dioxide nanosheets: from preparation to biomedical applications. Int J Nanomed 14:4781–4800. https://doi.org/10.2147/ijn.s207666
Sundari R, Alva S, Sebayang D, Wahyudi H, Jonit S, Kamaruddin A (2018) Characterization of fabricated MnO2-amberlite photocatalyst by FTIR, XRD and SEM for alizarin removal. IOP Conf Ser Mater Scince Eng 343:012003. https://doi.org/10.1088/1757-899X/343/1/012003
Zhou X, Shao C, Li X, Wang X, Guo X, Liu Y (2018) Three dimensional hierarchical heterostructures of g-C3N4 nanosheets/TiO2 nanofibers: controllable growth via gas-solid reaction and enhanced photocatalytic activity under visible light. J Hazard Mater 344:113–122. https://doi.org/10.1016/j.jhazmat.2017.10.006
Li Y, Li P, Wang J, Yang Y, Yao W, Wei Z, Wu J, Yan X et al (2018) Water soluble graphitic carbon nitride with tunable fluorescence for boosting broad-response photocatalysis. Appl Catal B 225:519–529. https://doi.org/10.1016/j.apcatb.2017.12.017
Feng X, Cox DF (2018) Oxidation of MnO(100) and NaMnO2 formation: Characterization of Mn2+ and Mn3+ surfaces via XPS and water TPD. Surf Sci 675:47–53. https://doi.org/10.1016/j.susc.2018.04.022
Wang Y, Zhu M, Jiang E, Hua R, Na R, Li QX (2017) A simple and rapid turn on ESIPT fluorescent probe for colorimetric and ratiometric detection of biothiols in living cells. Sci Rep 7(1):4377. https://doi.org/10.1038/s41598-017-03901-8
Cai QY, Li J, Ge J, Zhang L, Hu YL, Li ZH, Qu LB (2015) A rapid fluorescence “switch-on” assay for glutathione detection by using carbon dots-MnO2 nanocomposites. Biosens Bioelectron 72:31–36. https://doi.org/10.1016/j.bios.2015.04.077
Luo W, Zhang S, Meng Q, Zhou J, Jin R, Long X, Tang YP, Guo H (2021) A two-photon multi-emissive fluorescent probe for discrimination of Cys and Hcy/GSH via an aromatic substitution-rearrangement. Talanta 224:121833. https://doi.org/10.1016/j.talanta.2020.121833
Wang S, Wang M, Liu Y, Meng X, Ye Y, Song X, Liang Z (2021) Novel D-π-A conjugated microporous polymer as visible light-driven oxidase mimic for efficient colorimetric detection of glutathione. Sens Actuators B Chem 326:128808. https://doi.org/10.1016/j.snb.2020.128808
Li L, Shi L, Jia J, Eltayeb O, Lu W, Tang Y, Dong C, Shuang S (2020) Dual photoluminescence emission carbon dots for ratiometric fluorescent GSH sensing and cancer cell recognition. ACS Appl Mater Interfaces 12(16):18250–18257. https://doi.org/10.1021/acsami.0c00283
Li J, Jiao L, Xu W, Yan H, Chen G, Wu Y, Hu L, Gu W (2021) Cobalt oxyhydroxide nanosheets integrating with metal indicator enable sensitive detection of glutathione. Sens Actuators B Chem 329:129247. https://doi.org/10.1016/j.snb.2020.129247
Yan X, Song Y, Zhu C, Song J, Du D, Su X, Lin Y (2016) Graphene quantum dot-MnO2 nanosheet based optical sensing platform: a sensitive fluorescence “Turn Off-On” nanosensor for glutathione detection and intracellular imaging. ACS Appl Mater Interfaces 8(34):21990–21996. https://doi.org/10.1021/acsami.6b05465
Chen Y, Cong H, Shen Y, Yu B (2020) Biomedical application of manganese dioxide nanomaterials. Nanotechnology 31(20):202001. https://doi.org/10.1088/1361-6528/ab6fe1
Acknowledgements
Financial support from the Natural Science Foundation of Shanxi Province of China (201901D111210), Key Research Project of Science and Technology in JinZhong-Social Development Projects (Y213003), Special Project of Lvliang for Introduced High-level Science and Technology Talents (2021RC-2-33), National Natural Science Foundation of China (21874087), and Transverse Scientific Research Project of Shanxi University (2F022019056) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
CC and XY were involved in the conceptualization, methodology, investigation, formal analysis, data curation, and writing—original draft preparation. XY contributed to the resources, visualization, and investigation. CD contributed to the resources, and visualization. WB was involved in the conceptualization, supervision, funding acquisition, validation, and writing—original draft preparation. MMFC contributed to the writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Andrea de Camargo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chai, C., Yang, X., Yang, X. et al. An ultrasensitive MnO2-S,O-doped g-C3N4 nanoprobe for “turn-on” detection of glutathione and cell imaging. J Mater Sci 57, 7909–7922 (2022). https://doi.org/10.1007/s10853-022-07160-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07160-5