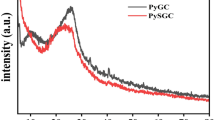
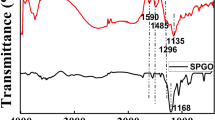
Abstract
Surfactant intercalated polypyrrole-exfoliated graphene oxide hybrid thin films were successfully electrodeposited as binder-free electrodes for supercapacitors. The superior electrochemical activity was achieved by tuning the specified morphology of the composite films through varying the concentration of the anionic surfactant sodium lauryl sulfate (SLS) during electrochemical deposition. Here, the surfactant acted as a stabilizing agent and a supporting electrolyte to achieve a uniform coating. Although all the composite films exhibited remarkable improvement in electrochemical performance upon sulfonation, the best performance was observed for 0.025 M SLS-derived electrodes. The fabricated symmetric supercapacitor (SC) achieved an outstanding specific capacitance of 494 F g−1 at 1 A g−1, with a specific energy of 17.1 W h kg−1 at 325 W kg−1 specific power and maintained 99% coulombic efficiency over 5000 GCD.
Graphical abstract








Similar content being viewed by others
References
Raza W, Ali F, Raza N, Luo Y, Kim KH, Yang J, Kwon EE (2018) Recent advancements in supercapacitor technology. Nano Energy 52:441–473. https://doi.org/10.1016/j.nanoen.2018.08.013
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41:797–828. https://doi.org/10.1039/c1cs15060j
Conway BE (1999) Electrochemical supercapacitors: scientific fundamentals and technological applications, 1st edn. Kluwer Academic/ Plenum, New York
Majumder M, Choudhary RB, Thakur AK, Karbhal I (2017) Impact of rare-earth metal oxide (Eu2O3) on the electrochemical properties of a polypyrrole/CuO polymeric composite for supercapacitor applications. RSC Adv 7:20037–20048. https://doi.org/10.1039/c7ra01438d
Winter M, Brodd RJ (2004) What are batteries, fuel cells, and supercapacitors? Chem Rev 104:4245–4269. https://doi.org/10.1021/cr020730k
Fan LQ, Liu GJ, Wu JH, Liu L, Lin JM, Wei YL (2014) Asymmetric supercapacitor based on graphene oxide/polypyrrole composite and activated carbon electrodes. Electrochim Acta 137:26–33. https://doi.org/10.1016/j.electacta.2014.05.137
Gu Y, Fan QL, Huang JL, Geng CL, Lin JM, Huang ML, Wu JH (2019) N-doped reduced graphene oxide decorated NiSe2 nanoparticles for high-performance asymmetric supercapacitors. J Power Sources 425:60–68. https://doi.org/10.1016/j.jpowsour.2019.03.123
Dhawale DS, Mane GP, Joseph S, Talapaneni SN, Anand C, Mano A, Vinu A (2015) Cobalt oxide functionalized nanoporous carbon electrodes and their excellent supercapacitive performance. RSC Adv 5:13930–13940. https://doi.org/10.1039/C4RA14041A
Wang YL, Fan LQ, Sun SJ, Chen JJ, Wu ZX, Zhu TT, Wu JH (2022) Ti3C2Tx MXene supported SnO2 quantum dots with oxygen vacancies as anode for Li-ion capacitors. Chem Eng J 428:131993. https://doi.org/10.1016/j.cej.2021.131993
A. R. Athira, V. M. Vimuna, K. Vidya, T. S. Xavier, (2018) 2nd International Conference on Condensed Matter & Applied Physics, Bikaner, India, Nov 24–25, 2017, AIP Conference Proceedings1953, American Institute of Physics: New York https://doi.org/10.1063/1.5032476
Zhu L, Hao C, Wang X, Guo Y (2020) Fluffy cotton-Like GO/Zn–Co–Ni layered double hydroxides form from a sacrificed template GO/ZIF-8 for high performance asymmetric supercapacitors. ACS Sustain Chem Eng 8:11618–11629. https://doi.org/10.1021/acssuschemeng.0c02916
Huang C, Ding Y, Hao C, Zhou S, Wang X, Gao H, Wu J (2019) PVP-assisted growth of Ni-Co oxide on N-doped reduced graphene oxide with enhanced pseudocapacitive behavior. Chem Eng J 378:122202. https://doi.org/10.1016/j.cej.2019.122202
Fong KD, Wang T (2017) Multidimensional performance optimization of Conducting polymer-based supercapacitor electrodes, Sustain. Energy Fuels 1:1857–1874. https://doi.org/10.1039/C7SE00339K
Horn MR, Williams F, Dubal D, MacLeod J, Motta N (2020) Simple method for estimating the surface area of layered graphene-based thin films. Chemsuschem 13:1613–1620. https://doi.org/10.1002/cssc.201901928
Zhou Q, Zhu D, Ma X, Mo D, Jiang F, Xu J, Zhou W (2016) PEDOT: PSS-assisted polyindole hollow nanospheres modified carbon cloth as high performance electrochemical capacitor electrodes. Electrochim Acta 212:662–670. https://doi.org/10.1016/j.electacta.2016.07.064
Huang Y, Li H, Wang Z, Zhu M, Pei Z, Xue Q, Zhi C (2016) Nanostructured polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 22:422–438. https://doi.org/10.1016/j.nanoen.2016.02.047
Yussuf A, Al-Saleh M, Al-Enezi S, Abraham G (2018) Synthesis, characterization of conductive polypyrrole: the influence of the oxidants and monomer on the electrical, thermal, and morphological properties. Int J Polym Sci 8:4191747. https://doi.org/10.1155/2018/4191747
Jiang Y, Hu C, Cheng H, Li C, Xu T, Zhao Y, Shao H, Qu L (2016) Spontaneous, straightforward fabrication of partially reduced graphene oxide–polypyrrole composite films for versatile actuators. ACS Nano 10:4735–4741. https://doi.org/10.1021/acsnano.6b01233
Patil DS, Shaikh JS, Dalavi DS, Kalagi SS, Patil PS (2011) Chemical synthesis of highly stable PVA/PANI films for supercapacitor application. Mater Chem Phys 128:449–455. https://doi.org/10.1016/j.matchemphys.2011.03.029
Fu SL, Zhou Q, Wu Y (2017) Latest advances in supercapacitors: from new electrode materials to novel device designs. Chem Soc Rev 46:6816–6854. https://doi.org/10.1039/c7cs00205j
Zhao C, Zheng W, Wang X, Zhang H, Cui X, Wang H (2013) Ultrahigh capacitive performance from both Co(OH) 2 /graphene electrode and K 3 Fe(CN) 6 electrolyte. Sci Rep 3:3–8. https://doi.org/10.1038/srep02986
Jiao S, Li T, Zhang Y, Xiong C, Zhao T (2016) A three-dimensional vertically aligned carbon nanotube /polyaniline composite as a supercapacitor electrode. RSC Adv 6:110592–110599. https://doi.org/10.1039/C6RA17674G
Zhou H, Han G, Xiao Y, Chang Y, Zhai HJ (2014) Facile preparation of polypyrrole/graphene oxide nanocomposites with large areal capacitance using electrochemical codeposition for supercapacitors. J Power Sources 263:259–267. https://doi.org/10.1016/j.jpowsour.2014.04.039
Shinde SS, Gund GS, Dubal DP, Jambure SB, Lokhande CD (2014) Morphological modulation of polypyrrole thin films through oxidizing agents and their concurrent effect on supercapacitor performance. Electrochim Acta 119:1–10. https://doi.org/10.1016/j.electacta.2013.10.174
Barakzehi M, Montazer M, Sharif F, Norby T, Chatzitakis A (2019) A textile-based wearable supercapacitor using reduced graphene oxide/polypyrrole composite. Electrochim Acta 305:187–196. https://doi.org/10.1016/j.electacta.2019.03.058
Pruna AI, Rosas-Laverde NM, Busquets Mataix D (2020) Effect of deposition parameters on electrochemical properties of polypyrrole-graphene oxide films. Materials 13:1–12. https://doi.org/10.3390/ma13030624
Kulandaivalu S, Suhaimi N, Sulaiman Y (2019) Unveiling high specific energy supercapacitor from layer-by-layer assembled polypyrrole/graphene oxide|polypyrrole/manganese oxide electrode material. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-41203-3
Biswas S, Drzal LT (2010) Multilayered nanoarchitecture of graphene nanosheets and polypyrrole nanowires for high performance supercapacitor electrodes. Chem Mater 22:5667–5671. https://doi.org/10.1021/cm101132g
Chen J, Wang Y, Cao J, Liu Y, Zhou Y, Ouyang JH, Jia D (2017) facile co-electrodeposition method for high-performance supercapacitor based on reduced graphene oxide/polypyrrole composite film. ACS Appl Mater Interfaces 9:19831–19842. https://doi.org/10.1021/acsami.7b03786
Ning D, Zhang A, Murtaza M, Wu H (2019) Effect of surfactants on the electrodeposition of Cu-TiO2 composite coatings prepared by jet electrodeposition. J Alloys Compd 777:1245–1250. https://doi.org/10.1016/j.jallcom.2018.11.077
Wei D, Zhu J, Luo L, Huang H, Li L, Yu X (2020) Fabrication of poly (vinyl alcohol)–graphene oxide–polypyrrole composite hydrogel for elastic supercapacitors. J Mater Sci 55:11779–11791. https://doi.org/10.1007/s10853-020-04833-x
Athira AR, Deepthi S, Xavier TS (2021) Impact of an anionic surfactant on the enhancement of the capacitance characteristics of polyaniline-wrapped graphene oxide hybrid composite. Bull Mater Sci 44:1–10. https://doi.org/10.1007/s12034-021-02481-8
Sarmah D, Kumar A (2019) Ion beam modified molybdenum disulfide-reduced graphene oxide/polypyrrole nanotubes ternary nanocomposite for hybrid supercapacitor electrode. Electrochim Acta 312:392–410. https://doi.org/10.1016/j.electacta.2019.04.174
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339–1339. https://doi.org/10.1021/ja01539a017
Zhou W, Ma X, Jiang F, Zhu D, Xu J, Lu B, Liu C (2014) Electrochemical fabrication of a porous network MnO2/poly(5-cyanoindole) composite and its capacitance performance. Electrochim Acta 138:270–277. https://doi.org/10.1016/j.electacta.2014.06.123
Wu J, Zhou W, Jiang F, Chang Y, Zhou Q, Li D, Du Y (2018) Three-dimensional porous carbon derived from polyindole hollow nanospheres for high-performance supercapacitor electrode. ACS Appl Energy Mater 1:4572–4579. https://doi.org/10.1021/acsaem.8b00722
Tong L, Gao M, Jiang C, Cai K (2019) Ultra-high performance and flexible polypyrrole coated CNT paper electrodes for all-solid-state supercapacitors. J Mater Chem A 7:10751–10760. https://doi.org/10.1039/C9TA01856E
Zhu C, Zhai J, Wen D, Dong S (2012) Graphene oxide/polypyrrole nanocomposites: one-step electrochemical doping, coating and synergistic effect for energy storage. J Mater Chem 22:6300. https://doi.org/10.1039/c2jm16699b
Li H, Liu L, Yang F (2013) Covalent assembly of 3D graphene/polypyrrole foams for oil spill cleanup. J Mater Chem A 1:3446–3453. https://doi.org/10.1039/c3ta00166k
Robinson BJ, Bailey SWD, O’Driscoll LJ, Visontai D, Welsh DJ, Mostert AB, Lambert C (2017) Formation of two-dimensional micelles on graphene: multi-scale theoretical and experimental study. ACS Nano 11:3404–3412. https://doi.org/10.1021/acsnano.7b01071
Politi S, Carcione R, Tamburri E, Matassa R, Lavecchia T, Angjellari M, Terranova ML (2018) Graphene platelets from shungite rock modulate electropolymerization and charge storage mechanisms of soft-template synthetized polypyrrole-based nanocomposites. Sci Rep 8:1–18. https://doi.org/10.1038/s41598-018-35415-2
Wang L, Liu F, Jin C, Zhang T, Yin Q (2014) Preparation of polypyrrole/graphene nanosheets composites with enhanced thermoelectric properties. RSC Adv 4:46187–46193. https://doi.org/10.1039/c4ra07774a
Cao J, Wang Y, Chen J, Li X, Walsh FC, Ouyang JH, Zhou Y (2015) Three-dimensional graphene oxide/polypyrrole composite electrodes fabricated by one-step electrodeposition for high performance supercapacitors. J Mater Chem A 3:14445–14457. https://doi.org/10.1039/C5TA02920A
Paulson BM, Joby TK, Raphael VP, Shaju KS (2018) Prevention of reinforcement corrosion in concrete by sodium lauryl sulphate: electrochemical and gravimetric investigations. Int J Corros 10:9471694. https://doi.org/10.1155/2018/9471694
Jiang Y, Hu C, Cheng H, Li C, Xu T, Zhao Y, Qu L (2016) Spontaneous, straightforward fabrication of partially reduced graphene oxide-polypyrrole composite films for versatile actuators. ACS Nano 10:4735–4741. https://doi.org/10.1021/acsnano.6b01233
Zhou Q, Zhu D, Ma X, Xu J, Zhou W, Zhao F (2016) High-performance capacitive behavior of layered reduced graphene oxide and polyindole nanocomposite materials. RSC Adv 6:29840–29847. https://doi.org/10.1039/C5RA27375G
Wang Y, Yang J, Wang L, Du K, Yin Q, Yin Q (2017) Polypyrrole/graphene/polyaniline ternary nanocomposite with high thermoelectric power factor. ACS Appl Mater Interfaces 9:20124–20131. https://doi.org/10.1021/acsami.7b05357
Xu C, Puente-Santiago AR, Rodriguez-Padron D, Caballero A, Balu AM, Romero AA, Luque R (2019) Controllable design of polypyrrole-iron oxide nanocoral architectures for supercapacitors with ultrahigh cycling stability. ACS Appl Energy Mater 2:2161–2168. https://doi.org/10.1021/acsaem.8b02167
Liu A, Li C, Bai H, Shi G (2010) Electrochemical deposition of polypyrrole/sulfonated graphene composite films. J Phys Chem C 114:22783–22789. https://doi.org/10.1021/jp108826e
Singh AK, Sarkar D, Karmakar K, Mandal K, Khan GG (2016) High-performance supercapacitor electrode based on cobalt oxide-manganese dioxide-nickel oxide ternary 1D hybrid nanotubes. ACS Appl Mater Interfaces 8:20786–20792. https://doi.org/10.1021/acsami.6b05933
Li GR, Feng ZP, Zhong JH, Wang ZL, Tong YX (2010) Electrochemical synthesis of polyaniline nanobelts with predominant electrochemical performances. Macromolecules 43:2178–2183. https://doi.org/10.1021/ma902317k
Chen W, Rakhi RB, Alshareef HN (2013) Morphology-dependent enhancement of the pseudocapacitance of template-guided tunable polyaniline nanostructures. J Phys Chem C 117:15009–15019. https://doi.org/10.1021/jp405300p
Grote F, Lei Y (2014) A complete three-dimensionally nanostructured asymmetric supercapacitor with high operating voltage window based on PPy and MnO2. Nano Energy 10:63–70. https://doi.org/10.1016/j.nanoen.2014.08.019
Luo J, Ma Q, Gu H, Zheng Y, Liu X (2015) Three-dimensional graphene-polyaniline hybrid hollow spheres by layer-by-layer assembly for application in supercapacitor. Electrochim Acta 173:184–192. https://doi.org/10.1016/j.electacta.2015.05.053
Liang A, Li D, Zhou W, Wu Y, Ye G, Wu J, Du Y (2018) Robust flexible WS2/PEDOT:PSS film for use in high-performance miniature supercapacitors. J Electroanal Chem 824:136–146. https://doi.org/10.1016/j.jelechem.2018.07.040
Liang A, Zhang Y, Jiang F, Zhou W, Xu J, Hou J, Duan X (2019) Electrochemical self-assembly of a 3D interpenetrating porous network PEDOT-PEG-WS2 nanocomposite for high-efficient energy storage. J Phys Chem C 123:25428–25436. https://doi.org/10.1021/acs.jpcc.9b05227
Zhou W, Xu J (2016) High-operating-voltage all-solid-state symmetrical supercapacitors based on poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate) films treated by organic solvents. Electrochim Acta 222:1895–1902. https://doi.org/10.1016/j.electacta.2016.11.181
Bello A, Barzegar F, Madito MJ, Momodu DY, Khaleed AA, Masikhwa TM, Manyala N (2016) Electrochemical performance of polypyrrole derived porous activated carbon-based symmetric supercapacitors in various electrolytes. RSC Adv 6:68141–68149. https://doi.org/10.1039/c6ra12690a
Zhou H, Zhai HJ, Zhi X (2018) Enhanced electrochemical performances of polypyrrole/carboxyl graphene/carbon nanotubes ternary composite for supercapacitors. Electrochim Acta 290:1–11. https://doi.org/10.1016/j.electacta.2018.09.039
Asen P, Shahrokhian S (2017) A high performance supercapacitor based on graphene/polypyrrole/Cu2O–Cu (OH) 2 ternary nanocomposite coated on nickel foam. J Phys Chem C 121:6508–6519. https://doi.org/10.1021/acs.jpcc.7b00534
Wang W, Sadak O, Guan J, Gunasekaran S (2020) Facile synthesis of graphene paper/polypyrrole nanocomposite as electrode for flexible solid-state supercapacitor. J Energy Storage 30:101533. https://doi.org/10.1016/j.est.2020.101533
Yang L, Shi M, Jiang J, Liu Y, Yan C, Liu H, Guo Z (2019) Heterogeneous interface induced formation of balsam pear-like PPy for high performance supercapacitors. Mater Lett 244:27–30. https://doi.org/10.1016/j.matlet.2019.02.064
Prasankumar T, Vigneshwaran J, Abraham S, Jose SP (2018) 3D structures of graphene oxide and graphene analogue MoS2 with polypyrrole for supercapacitor electrodes. Mater Lett 238:121–125. https://doi.org/10.1016/j.matlet.2018.12.002
AlDream J, Zequine C, Siam K, Kahol PK, Mishra SR, Gupta RK (2019) Electrochemical properties of graphene oxide nanoribbons/polypyrrole nanocomposites. C 5(2):18
Mondal S, Rana U, Malik S (2017) Reduced graphene Oxide/Fe3O4/polyaniline nanostructures as electrode materials for an all-solid-state hybrid supercapacitor. J Phys Chem C 121(14):7573–7583. https://doi.org/10.1021/acs.jpcc.6b10978
Acknowledgements
The authors are thankful to DST-FIST, State Centre for Advanced Instrumentation, Govt. College for Women, Kerala Government project "Performance Linked Encouragement for Academic Studies (PLEASE)," and the Department of Optoelectronics for analysis support. One of the authors (1A R Athira) acknowledges the University of Kerala for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Handling Editor: Joshua Tong.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Athira, A.R., Vimuna, V.M., Tomy, M. et al. Surfactant intercalated polypyrrole-exfoliated graphene oxide hybrid thin film symmetric supercapacitor. J Mater Sci 57, 6749–6762 (2022). https://doi.org/10.1007/s10853-022-07075-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07075-1