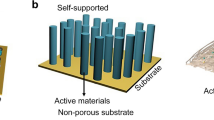
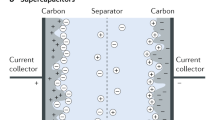
Abstract
Electrochemical energy storage is becoming more ubiquitous in the world, and with that comes an urgent need for increased performance. One promising approach in the pursuit of next-generation energy storage with simultaneous high energy and high power is through cooperative assembly of electrochemically active materials into conductive scaffolds. In such architectures, the active material is often directly bonded to the conductive scaffold, therefore reducing the need for separate binders and current collectors. The conductive scaffold material can also provide a robust, free-standing structure that is capable of enduring mechanical deformation, which is particularly important for high gravimetric capacity materials that can undergo significant volume changes during electrochemical cycling. This review summarizes several of the most common approaches for developing free-standing binder-free electrode architectures of transition metal oxides that aim to achieve simultaneous high energy and high power for the next generation of electrochemical energy storage devices.










Similar content being viewed by others
References
Dunn B, Kamath H, Tarascon J-M (2011) Electrical energy storage for the grid: a battery of choices. Science 334:928–935. https://doi.org/10.1126/science.1212741
U.S. Energy Information Administration (2018) Electricity in the United States. https://www.eia.gov/energyexplained/index.php?page=electricity_in_the_united_states. Accessed 2 Mar 2019
Schmuch R, Wagner R, Hörpel G, Placke T, Winter M (2018) Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat Energy 3:267–278. https://doi.org/10.1038/s41560-018-0107-2
Cano ZP, Banham D, Ye S, Hintennach A, Lu J, Fowler M, Chen Z (2018) Batteries and fuel cells for emerging electric vehicle markets. Nat Energy 3:279–289. https://doi.org/10.1038/s41560-018-0108-1
Cox B, Mutel CL, Bauer C, Mendoza Beltran A, van Vuuren DP (2018) Uncertain environmental footprint of current and future battery electric vehicles. Environ Sci Technol 52:4989–4995. https://doi.org/10.1021/acs.est.8b00261
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22:587–603. https://doi.org/10.1021/cm901452z
Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D (2011) Challenges in the development of advanced Li-ion batteries: a review. Energy Environ Sci 4:3243. https://doi.org/10.1039/c1ee01598b
Turcheniuk K, Bondarev D, Singhal V, Yushin G (2018) Ten years left to redesign lithium-ion batteries. Nature 559:467–470. https://doi.org/10.1038/d41586-018-05752-3
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7:845–854. https://doi.org/10.1038/nmat2297
Cheng F, Liang J, Tao Z, Chen J (2011) Functional materials for rechargeable batteries. Adv Mater 23:1695–1715. https://doi.org/10.1002/adma.201003587
Whittingham MS (2004) Lithium batteries and cathode materials. Chem Rev 104:4271–4302. https://doi.org/10.1021/cr020731c
Liu J, Xu C, Chen Z, Ni S, Shen ZX (2018) Progress in aqueous rechargeable batteries. Green Energy Environ 3:20–41. https://doi.org/10.1016/j.gee.2017.10.001
Kraytsberg A, Ein-Eli Y (2016) Conveying advanced Li-ion battery materials into practice the impact of electrode slurry preparation skills. Adv Energy Mater 6:1600655. https://doi.org/10.1002/aenm.201600655
Tao B, Yule LC, Daviddi E, Bentley CL, Unwin PR (2019) Correlative electrochemical microscopy of Li-ion (De)intercalation at a series of individual LiMn2O4 particles. Angew Chemie Int Ed 58:4606–4611. https://doi.org/10.1002/anie.201814505
Braun PV, Cook JB (2018) Deterministic design of chemistry and mesostructure in Li-ion battery electrodes. ACS Nano 12:3060–3064. https://doi.org/10.1021/acsnano.8b01885
Department of energy (2017) Basic research needs for next generation electrical energy storage. https://science.osti.gov/bes/Community-Resources/Reports
Long JW, Dunn B, Rolison DR, White HS (2004) Three-dimensional battery architectures. Chem Rev 104:4463–4492. https://doi.org/10.1021/cr020740l
Long JW, Rolison DR (2007) Architectural design, interior decoration, and three-dimensional plumbing en route to multifunctional nanoarchitectures. Acc Chem Res 40:854–862. https://doi.org/10.1021/ar6000445
Xia X, Zhang YY, Chao D et al (2014) Solution synthesis of metal oxides for electrochemical energy storage applications. Nanoscale 6:5008–5048. https://doi.org/10.1039/C4NR00024B
Zhang H, Ning H, Busbee J et al (2017) Electroplating lithium transition metal oxides. Sci Adv 3:e1602427. https://doi.org/10.1126/sciadv.1602427
Elam JW, Dasgupta NP, Prinz FB (2011) ALD for clean energy conversion, utilization, and storage. MRS Bull 36:899–906. https://doi.org/10.1557/mrs.2011.265
Dasgupta NP, Sun J, Liu C et al (2014) 25th Anniversary article: semiconductor nanowires—synthesis, characterization, and applications. Adv Mater 26:2137–2183. https://doi.org/10.1002/adma.201305929
Zheng J, Zhao Q, Liu X et al (2019) Nonplanar electrode architectures for ultrahigh areal capacity batteries. ACS Energy Lett 4:271–275. https://doi.org/10.1021/acsenergylett.8b02131
Rolison DR, Long JW, Lytle JC, Fischer AE, Rhodes CP, McEvoy TM, Bourg ME, Lubers AM (2009) Multifunctional 3D nanoarchitectures for energy storage and conversion. Chem Soc Rev 38:226–252. https://doi.org/10.1039/B801151F
Sherrill SA, Banerjee P, Rubloff GW, Lee SB (2011) High to ultra-high power electrical energy storage. Phys Chem Chem Phys 13:20714–20723. https://doi.org/10.1039/c1cp22659b
Lukatskaya MR, Dunn B, Gogotsi Y (2016) Multidimensional materials and device architectures for future hybrid energy storage. Nat Commun 7:1–13. https://doi.org/10.1038/ncomms12647
Sun H, Zhu J, Baumann D, Peng L, Xu Y, Shakir I, Huang Y, Duan X (2019) Hierarchical 3D electrodes for electrochemical energy storage. Nat Rev Mater 4:45–60. https://doi.org/10.1038/s41578-018-0069-9
Kang J, Zhang S, Zhang Z (2017) Three-dimensional binder-free nanoarchitectures for advanced pseudocapacitors. Adv Mater 29:1700515. https://doi.org/10.1002/adma.201700515
Manthiram A (2003) Materials aspects: an overview. In: Nazri GA, Pistoia G (eds) Lithium batteries: science and technology. Springer, Berlin, pp 3–41
Augustyn V, Simon P, Dunn B (2014) Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ Sci 7:1597. https://doi.org/10.1039/c3ee44164d
Whittingham M (2000) Insertion electrodes as SMART materials: the first 25 years and future promises. Solid State Ionics 134:169–178. https://doi.org/10.1016/S0167-2738(00)00724-4
Cabana J, Monconduit L, Larcher D, Palacín MR (2010) Beyond intercalation-based Li-ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions. Adv Mater 22:E170–E192. https://doi.org/10.1002/adma.201000717
Whittingham MS (2004) Hydrogen motion in oxides: from insulators to bronzes. Solid State Ionics 168:255–263. https://doi.org/10.1016/j.ssi.2003.08.056
Yang Y, Liu Q, Cao M, Ju Q, Wang H, Fu R, Ji H, Yang G (2019) Enhanced electrochemical performance of α-Fe2O3 grains grafted onto TiO2–carbon nanofibers via a vapor–solid reaction as anode materials for Li-Ion batteries. Appl Surf Sci 463:322–330. https://doi.org/10.1016/j.apsusc.2018.08.171
Huang Z-H, Song Y, Feng D-Y, Sun Z, Sun X, Liu X-X (2018) High mass loading MnO2 with hierarchical nanostructures for supercapacitors. ACS Nano 12:3557–3567. https://doi.org/10.1021/acsnano.8b00621
Wu C, Zhu Y, Ding M, Jia C, Zhang K (2018) Fabrication of plate-like MnO2 with excellent cycle stability for supercapacitor electrodes. Electrochim Acta 291:249–255. https://doi.org/10.1016/j.electacta.2018.08.126
Kim H-S, Cook JB, Lin H, Ko JS, Tolbert SH, Ozolins V, Dunn B (2017) Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x. Nat Mater 16:454–460. https://doi.org/10.1038/nmat4810
Augustyn V (2017) Tuning the interlayer of transition metal oxides for electrochemical energy storage. J Mater Res 32:2–15. https://doi.org/10.1557/jmr.2016.337
Li X, Liu J, Banis MN, Lushington A, Li R, Cai M, Sun X (2014) Atomic layer deposition of solid-state electrolyte coated cathode materials with superior high-voltage cycling behavior for lithium ion battery application. Energy Environ Sci 7:768–778. https://doi.org/10.1039/c3ee42704h
Wu Y, Wen Z, Li J (2011) Hierarchical carbon-coated LiFePO4 nanoplate microspheres with high electrochemical performance for Li-ion batteries. Adv Mater 23:1126–1129. https://doi.org/10.1002/adma.201003713
Liu T, Wang W, Yi M, Chen Q, Xu C, Cai D, Zhan H (2018) Metal-organic framework derived porous ternary ZnCo2O4 nanoplate arrays grown on carbon cloth as binder-free electrodes for lithium-ion batteries. Chem Eng J 354:454–462. https://doi.org/10.1016/j.cej.2018.08.037
Xia X, Tu J, Zhang Y, Wang X, Gu C, Zhao X-B, Fan HJ (2012) High-quality metal oxide core/shell nanowire arrays on conductive substrates for electrochemical energy storage. ACS Nano 6:5531–5538. https://doi.org/10.1021/nn301454q
Lim GJH, Liu X, Guan C, Wang J (2018) Co/Zn bimetallic oxides derived from metal organic frameworks for high performance electrochemical energy storage. Electrochim Acta 291:177–187. https://doi.org/10.1016/j.electacta.2018.08.105
Wang J, Dong L, Xu C, Ren D, Ma X, Kang F (2018) Polymorphous supercapacitors constructed from flexible three-dimensional carbon network/polyaniline/MnO2 composite textiles. ACS Appl Mater Interfaces 10:10851–10859. https://doi.org/10.1021/acsami.7b19195
Wu H, Xu M, Wang Y, Zheng G (2013) Branched Co3O4/Fe2O3 nanowires as high capacity lithium-ion battery anodes. Nano Res 6:167–173. https://doi.org/10.1007/s12274-013-0292-z
Fleischmann S, Zeiger M, Quade A, Kruth A, Presser V (2018) Atomic layer-deposited molybdenum oxide/carbon nanotube hybrid electrodes: the influence of crystal structure on lithium-ion capacitor performance. ACS Appl Mater Interfaces 10:18675–18684. https://doi.org/10.1021/acsami.8b03233
Wang L, Gu X, Zhao L, Wang B, Jia C, Xu J, Zhao Y, Zhang J (2019) ZnO@TiO2 heterostructure arrays/carbon cloth by charge redistribution enhances performance in flexible anode for Li ion batteries. Electrochim Acta 295:107–112. https://doi.org/10.1016/j.electacta.2018.10.146
Luo Y, Luo J, Jiang J et al (2012) Seed-assisted synthesis of highly ordered TiO2@α-Fe2O3 core/shell arrays on carbon textiles for lithium-ion battery applications. Energy Environ Sci 5:6559. https://doi.org/10.1039/c2ee03396h
Xu C, Li Z, Yang C et al (2016) An ultralong, highly oriented nickel-nanowire-array electrode scaffold for high-performance compressible pseudocapacitors. Adv Mater 28:4105–4110. https://doi.org/10.1002/adma.201505644
Li Y-Q, Li J-C, Lang X-Y, Wen Z, Zheng W-T, Jiang Q (2017) Lithium ion breathable electrodes with 3D hierarchical architecture for ultrastable and high-capacity lithium storage. Adv Funct Mater 27:1700447. https://doi.org/10.1002/adfm.201700447
Aricò AS, Bruce P, Scrosati B, Tarascon J-M, van Schalkwijk W (2005) Nanostructured materials for advanced energy conversion and storage devices. Nat Mater 4:366–377. https://doi.org/10.1038/nmat1368
Sun Y, Liu N, Cui Y (2016) Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat Energy 1:16071. https://doi.org/10.1038/nenergy.2016.71
Zhang F, Qi L (2016) Recent progress in self-supported metal oxide nanoarray electrodes for advanced lithium-ion batteries. Adv Sci 3:1600049. https://doi.org/10.1002/advs.201600049
Wang Y, Takahashi K, Shang H, Cao G (2005) Synthesis and electrochemical properties of vanadium pentoxide nanotube arrays. J Phys Chem B 109:3085–3088. https://doi.org/10.1021/jp044286w
Choudhary N, Li C, Chung H-S, Moore J, Thomas J, Jung Y (2016) High-performance one-body core/shell nanowire supercapacitor enabled by conformal growth of capacitive 2D WS2 layers. ACS Nano 10:10726–10735. https://doi.org/10.1021/acsnano.6b06111
Zhong Y, Ma Y, Guo Q, Liu J, Wang Y, Yang M, Xia H (2017) Controllable synthesis of TiO2@Fe2O3 core–shell nanotube arrays with double-wall coating as superb lithium-ion battery anodes. Sci Rep 7:40927. https://doi.org/10.1038/srep40927
Tang Y, Hong L, Wu Q, Li J, Hou G, Cao H, Wu L, Zheng G (2016) TiO2(B) nanowire arrays on Ti foil substrate as three-dimensional anode for lithium-ion batteries. Electrochim Acta 195:27–33. https://doi.org/10.1016/j.electacta.2016.01.235
Pawlitzek F, Althues H, Schumm B, Kaskel S (2017) Nanostructured networks for energy storage: vertically aligned carbon nanotubes (VACNT) as current collectors for high-power Li4Ti5O12(LTO)//LiMn2O4(LMO) lithium-ion batteries. Batteries 3:37. https://doi.org/10.3390/batteries3040037
Jhao J-J, Lin C-H, Yeh T-K, Wu H-C, Tsai M-C, Hsieh C-K (2017) The coaxial nanostructure of ruthenium oxide thin films coated onto the vertically grown graphitic nanofibers for electrochemical supercapacitor. Surf Coat Technol 320:263–269. https://doi.org/10.1016/j.surfcoat.2017.01.006
Zhang H, Yu X, Braun PV (2011) Three-dimensional bicontinuous ultrafast-charge and-discharge bulk battery electrodes. Nat Nanotechnol 6:277–281. https://doi.org/10.1038/nnano.2011.38
Wang J, Zhou H, Nanda J, Braun PV (2015) Three-dimensionally mesostructured Fe2O3 electrodes with good rate performance and reduced voltage hysteresis. Chem Mater 27:2803–2811. https://doi.org/10.1021/cm504365s
Liu J, Wang J, Kim J et al (2015) High full-electrode basis capacity template-free 3D nanocomposite secondary battery anodes. Small 11:6265–6271. https://doi.org/10.1002/smll.201502538
Liu J, Zheng Q, Goodman MD et al (2016) Graphene sandwiched mesostructured Li-ion battery electrodes. Adv Mater 28:7696–7702. https://doi.org/10.1002/adma.201600829
Hou C, Lang X-Y, Han G-F et al (2013) Integrated solid/nanoporous copper/oxide hybrid bulk electrodes for high-performance lithium-ion batteries. Sci Rep 3:2878. https://doi.org/10.1038/srep02878
Xiao S, Bi F, Zhao L, Wang L, Gai G (2017) Design and synthesis of H-TiO2/MnO2 core–shell nanotube arrays with high capacitance and cycling stability for supercapacitors. J Mater Sci 52:7744–7753. https://doi.org/10.1007/s10853-017-1034-5
Sathiya M, Prakash AS, Ramesha K, Tarascon J, Shukla AK (2011) V2O5-anchored carbon nanotubes for enhanced electrochemical energy storage. J Am Chem Soc 133:16291–16299. https://doi.org/10.1021/ja207285b
Wu Z-S, Zhou G, Yin L-C, Ren W, Li F, Cheng H-M (2012) Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 1:107–131. https://doi.org/10.1016/j.nanoen.2011.11.001
Xu Y, Lin Z, Zhong X, Huang X, Weiss NO, Huang Y, Duan X (2014) Holey graphene frameworks for highly efficient capacitive energy storage. Nat Commun 5:4554. https://doi.org/10.1038/ncomms5554
Xu Y, Chen C-Y, Zhao Z et al (2015) Solution processable holey graphene oxide and its derived macrostructures for high-performance supercapacitors. Nano Lett 15:4605–4610. https://doi.org/10.1021/acs.nanolett.5b01212
Sun H, Mei L, Liang J et al (2017) Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage. Science 356:599–604. https://doi.org/10.1126/science.aam5852
Chen X, Xiao T, Wang S, Li J, Xiang P, Jiang L, Tan X (2019) Superior Li-ion storage performance of graphene decorated NiO nanowalls on Ni as anode for lithium ion batteries. Mater Chem Phys 222:31–36. https://doi.org/10.1016/j.matchemphys.2018.09.061
Chen Y, Wang Y, Wang Z et al (2018) Densification by compaction as an effective low-cost method to attain a high areal lithium storage capacity in a CNT@Co3O4 sponge. Adv Energy Mater 8:1702981. https://doi.org/10.1002/aenm.201702981
Zhang Z, Zhang X, Feng Y et al (2018) Fabrication of porous ZnCo2O4 nanoribbon arrays on nickel foam for high-performance supercapacitors and lithium-ion batteries. Electrochim Acta 260:823–829. https://doi.org/10.1016/j.electacta.2017.12.047
Jadhav HS, Pawar SM, Jadhav AH, Thorat GM, Seo JG (2016) Hierarchical mesoporous 3D flower-like CuCo2O4/NF for high-performance electrochemical energy storage. Sci Rep 6:31120. https://doi.org/10.1038/srep31120
Zhou D, Cheng P, Luo J, Xu W, Li J, Yuan D (2017) Facile synthesis of graphene@NiMoO4 nanosheet arrays on Ni foam for a high-performance asymmetric supercapacitor. J Mater Sci 52:13909–13919. https://doi.org/10.1007/s10853-017-1467-x
Xia X, Chao D, Ng CF, Lin J, Fan Z, Zhang H, Shen ZX, Fan HJ (2015) VO2 nanoflake arrays for supercapacitor and Li-ion battery electrodes: performance enhancement by hydrogen molybdenum bronze as an efficient shell material. Mater Horizons 2:237–244. https://doi.org/10.1039/C4MH00212A
Lang X, Zhang H, Xue X et al (2018) Rational design of La0.85Sr0.15MnO3@NiCo2O4 core–shell architecture supported on Ni foam for high performance supercapacitors. J Power Sources 402:213–220. https://doi.org/10.1016/j.jpowsour.2018.09.040
Yuan J, Chen C, Hao Y et al (2017) Three-dimensionally porous CoMn2O4 thin films grown on Ni foams for high-performance lithium-ion battery anodes. J Mater Sci 52:5751–5758. https://doi.org/10.1007/s10853-017-0810-6
Li L, Zhang YQ, Liu XY et al (2014) One-dimension MnCo2O4 nanowire arrays for electrochemical energy storage. Electrochim Acta 116:467–474. https://doi.org/10.1016/j.electacta.2013.11.081
Wang J, Zhang H, Lv X, Nie K, Gao X, Zhong J, Sun X (2016) Self-supported ultrathin mesoporous CoFe2O4/CoO nanosheet arrays assembled from nanowires with enhanced lithium storage performance. J Mater Sci 51:6590–6599. https://doi.org/10.1007/s10853-016-9902-y
Zhang F, Yang C, Guan H, Hu Y, Jin C, Zhou H, Qi L (2018) 3D copper foam@FeOx nanoarrays as a high areal capacity and stable electrode for lithium-ion batteries. ACS Appl Energy Mater. https://doi.org/10.1021/acsaem.8b01024
Um JH, Choi M, Park H, Cho Y-H, Dunand DC, Choe H, Sung Y-E (2016) 3D macroporous electrode and high-performance in lithium-ion batteries using SnO2 coated on Cu foam. Sci Rep 6:18626. https://doi.org/10.1038/srep18626
Wang J, Wang G, Wang H (2015) Flexible free-standing Fe2O3/graphene/carbon nanotubes hybrid films as anode materials for high performance lithium-ion batteries. Electrochim Acta 182:192–201. https://doi.org/10.1016/j.electacta.2015.09.080
Xu Y, Xuan H, Gao J et al (2018) Hierarchical three-dimensional NiMoO4-anchored rGO/Ni foam as advanced electrode material with improved supercapacitor performance. J Mater Sci 53:8483–8498. https://doi.org/10.1007/s10853-018-2171-1
Zhang H, Deng X, Huang H et al (2018) Hetero-structure arrays of NiCoO2 nanoflakes@nanowires on 3D graphene/nickel foam for high-performance supercapacitors. Electrochim Acta 289:193–203. https://doi.org/10.1016/j.electacta.2018.08.071
Zhai X, Mao Z, Zhao G, Rooney D, Zhang N, Sun K (2018) Nanoflake δ-MnO2 deposited on carbon nanotubes-graphene-Ni foam scaffolds as self-standing three-dimensional porous anodes for high-rate-performance lithium-ion batteries. J Power Sources 402:373–380. https://doi.org/10.1016/j.jpowsour.2018.09.057
Jia H, Cai Y, Zheng X et al (2018) Mesostructured carbon nanotube-on-MnO2 nanosheet composite for high-performance supercapacitors. ACS Appl Mater Interfaces 10:38963–38969. https://doi.org/10.1021/acsami.8b14109
Xia X, Chao D, Zhang Y et al (2016) Generic synthesis of carbon nanotube branches on metal oxide arrays exhibiting stable high-rate and long-cycle sodium-ion storage. Small 12:3048–3058. https://doi.org/10.1002/smll.201600633
Chao D, Xia X, Liu J et al (2014) A V2O5/conductive-polymer core/shell nanobelt array on three-dimensional graphite foam: a high-rate, ultrastable, and freestanding cathode for lithium-ion batteries. Adv Mater 26:5794–5800. https://doi.org/10.1002/adma.201400719
Lin L, Tang S, Zhao S, Peng X, Hu N (2017) Hierarchical three-dimensional FeCo2O4@MnO2 core–shell nanosheet arrays on nickel foam for high-performance supercapacitor. Electrochim Acta 228:175–182. https://doi.org/10.1016/j.electacta.2017.01.022
Luo J, Xia X, Luo Y et al (2013) Rationally designed hierarchical TiO2@Fe2O3 hollow nanostructures for improved lithium ion storage. Adv Energy Mater 3:737–743. https://doi.org/10.1002/aenm.201200953
Wang B, Li S, Wu X, Liu J, Tian W (2016) Hierarchical NiMoO4 nanowire arrays supported on macroporous graphene foam as binder-free 3D anodes for high-performance lithium storage. Phys Chem Chem Phys 18:908–915. https://doi.org/10.1039/C5CP04820F
Chen X, Zhu H, Chen Y-C, Shang Y, Cao A, Hu L, Rubloff GW (2012) MWCNT/V2O5 core/shell sponge for high areal capacity and power density li-ion cathodes. ACS Nano 6:7948–7955. https://doi.org/10.1021/nn302417x
Ko JS, Sassin MB, Parker JF, Rolison DR, Long JW (2018) Combining battery-like and pseudocapacitive charge storage in 3D MnOx@carbon electrode architectures for zinc-ion cells. Sustain Energy Fuels 2:626–636. https://doi.org/10.1039/C7SE00540G
Guan C, Liu J, Wang Y, Mao L, Fan Z, Shen Z, Zhang H, Wang J (2015) Iron oxide-decorated carbon for supercapacitor anodes with ultrahigh energy density and outstanding cycling stability. ACS Nano 9:5198–5207. https://doi.org/10.1021/acsnano.5b00582
Liu B, Zhang J, Wang X, Chen G, Chen D, Zhou C, Shen G (2012) Hierarchical three-dimensional ZnCo2O4 nanowire arrays/carbon cloth anodes for a novel class of high-performance flexible lithium-ion batteries. Nano Lett 12:3005–3011. https://doi.org/10.1021/nl300794f
Huang Z-H, Song Y, Liu X-X (2019) Boosting operating voltage of vanadium oxide-based symmetric aqueous supercapacitor to 2 V. Chem Eng J 358:1529–1538. https://doi.org/10.1016/j.cej.2018.10.136
Ma L, Fan H, Wei X et al (2018) Towards high areal capacitance, rate capability, and tailorable supercapacitors: Co3O4@polypyrrole core–shell nanorod bundle array electrodes. J Mater Chem A 6:19058–19065. https://doi.org/10.1039/C8TA07477A
Qu G, Cheng J, Li X, Yuan D, Chen P, Chen X, Wang B, Peng H (2016) A fiber supercapacitor with high energy density based on hollow graphene/conducting polymer fiber electrode. Adv Mater 28:3646–3652. https://doi.org/10.1002/adma.201600689
Noh J, Yoon C-M, Kim YK, Jang J (2017) High performance asymmetric supercapacitor twisted from carbon fiber/MnO2 and carbon fiber/MoO3. Carbon 116:470–478. https://doi.org/10.1016/j.carbon.2017.02.033
Shah HU, Wang F, Javed MS, Ahmad MA, Saleem M, Zhan J, Khan ZUH, Li Y (2018) In-situ growth of MnO2 nanorods forest on carbon textile as efficient electrode material for supercapacitors. J Energy Storage 17:318–326. https://doi.org/10.1016/j.est.2018.03.015
Zhu Y, Huang Y, Wang M, Wang K, Yu M, Chen X, Zhang Z (2018) Novel carbon coated core–shell heterostructure NiCo2O4@NiO grown on carbon cloth as flexible lithium-ion battery anodes. Ceram Int 44:21690–21698. https://doi.org/10.1016/j.ceramint.2018.08.257
Zhang L, Zhang Y, Huang S et al (2018) Co3O4/Ni-based MOFs on carbon cloth for flexible alkaline battery-supercapacitor hybrid devices and near-infrared photocatalytic hydrogen evolution. Electrochim Acta 281:189–197. https://doi.org/10.1016/j.electacta.2018.05.162
Shen L, Che Q, Li H, Zhang X (2014) Mesoporous NiCo2O4 nanowire arrays grown on carbon textiles as binder-free flexible electrodes for energy storage. Adv Funct Mater 24:2630–2637. https://doi.org/10.1002/adfm.201303138
Zhu C, Usiskin RE, Yu Y, Maier J (2017) The nanoscale circuitry of battery electrodes. Science 358:eaao2808. https://doi.org/10.1126/science.aao2808
Wang R, Xu C, Sun J, Gao L, Lin C (2013) Flexible free-standing hollow Fe3O4/graphene hybrid films for lithium-ion batteries. J Mater Chem A 1:1794–1800. https://doi.org/10.1039/C2TA00753C
Zhou G, Wang D-W, Hou P-X, Li W, Li N, Liu C, Li F, Cheng H-M (2012) A nanosized Fe2O3 decorated single-walled carbon nanotube membrane as a high-performance flexible anode for lithium ion batteries. J Mater Chem 22:17942. https://doi.org/10.1039/c2jm32893c
Haichao L, Haoyi L, Bubakir MM, Weimin Y, Barhoum A (2018) Engineering nanofibers as electrode and membrane materials for batteries, supercapacitors, and fuel cells. In: Barhoum A, Bechelany M, Makhlouf A (eds) Handbook of nanofibers. Springer, Cham, pp 1–27
Liu X, Jiang Y, Li K, Xu F, Zhang P, Ding Y (2019) Electrospun free-standing N-doped C@SnO2 anode paper for flexible Li-ion batteries. Mater Res Bull 109:41–48. https://doi.org/10.1016/j.materresbull.2018.09.023
Cuan J, Zhang F, Zhang H et al (2018) Heterostructure manipulation toward ameliorating electrodes for better lithium storage capability. ACS Sustain Chem Eng 6:17267–17276. https://doi.org/10.1021/acssuschemeng.8b04685
Kong D, Li X, Zhang Y et al (2016) Encapsulating V2O5 into carbon nanotubes enables the synthesis of flexible high-performance lithium ion batteries. Energy Environ Sci 9:906–911. https://doi.org/10.1039/C5EE03345D
Rolison DR, Dunn B (2001) Electrically conductive oxide aerogels: new materials in electrochemistry. J Mater Chem 11:963–980. https://doi.org/10.1039/b007591o
Hong J-Y, Bak BM, Wie JJ, Kong J, Park HS (2015) Reversibly compressible, highly elastic, and durable graphene aerogels for energy storage devices under limiting conditions. Adv Funct Mater 25:1053–1062. https://doi.org/10.1002/adfm.201403273
Chen D, Peng L, Yuan Y et al (2017) Two-dimensional holey Co3O4 nanosheets for high-rate alkali-ion batteries: from rational synthesis to in situ probing. Nano Lett 17:3907–3913. https://doi.org/10.1021/acs.nanolett.7b01485
Liu L, Yang X, Lv C, Zhu A, Zhu X, Guo S, Chen C, Yang D (2016) Seaweed-derived route to Fe2O3 hollow nanoparticles/N-doped graphene aerogels with high lithium ion storage performance. ACS Appl Mater Interfaces 8:7047–7053. https://doi.org/10.1021/acsami.5b12427
Huang H, Wang X, Tervoort E, Zeng G, Liu T, Chen X, Sologubenko A, Niederberger M (2018) Nano-sized structurally disordered metal oxide composite aerogels as high-power anodes in hybrid supercapacitors. ACS Nano 12:2753–2763. https://doi.org/10.1021/acsnano.7b09062
Sun W, Gao G, Zhang K, Liu Y, Wu G (2018) Self-assembled 3D N-CNFs/V2O5 aerogels with core/shell nanostructures through vacancies control and seeds growth as an outstanding supercapacitor electrode material. Carbon 132:667–677. https://doi.org/10.1016/j.carbon.2018.03.004
Wang R, Xu C, Sun J, Gao L, Yao H (2014) Solvothermal-induced 3D macroscopic SnO2/nitrogen-doped graphene aerogels for high capacity and long-life lithium storage. ACS Appl Mater Interfaces 6:3427–3436. https://doi.org/10.1021/am405557c
Zhou Y, Wen L, Zhan K, Yan Y, Zhao B (2018) Three-dimensional porous graphene/nickel cobalt mixed oxide composites for high-performance hybrid supercapacitor. Ceram Int 44:21848–21854. https://doi.org/10.1016/j.ceramint.2018.08.292
Liu M, Liu Y, Zhang Y, Li Y, Zhang P, Yan Y, Liu T (2016) Octahedral tin dioxide nanocrystals anchored on vertically aligned carbon aerogels as high capacity anode materials for lithium-ion batteries. Sci Rep 6:31496. https://doi.org/10.1038/srep31496
Gao X-T, Liu Y-T, Zhu X-D, Yan D-J, Wang C, Feng Y-J, Sun K-N (2018) V2O5 nanoparticles confined in three-dimensionally organized, porous nitrogen-doped graphene frameworks: flexible and free-standing cathodes for high performance lithium storage. Carbon 140:218–226. https://doi.org/10.1016/j.carbon.2018.08.060
Yao B, Chandrasekaran S, Zhang J et al (2019) Efficient 3D printed pseudocapacitive electrodes with ultrahigh MnO2 loading. Joule 3:459–470. https://doi.org/10.1016/j.joule.2018.09.020
Takada K (2013) Progress and prospective of solid-state lithium batteries. Acta Mater 61:759–770. https://doi.org/10.1016/j.actamat.2012.10.034
Manthiram A, Yu X, Wang S (2017) Lithium battery chemistries enabled by solid-state electrolytes. Nat Rev Mater 2:16103. https://doi.org/10.1038/natrevmats.2016.103
Zhang B, Tan R, Yang L, Zheng J, Zhang K, Mo S, Lin Z, Pan F (2018) Mechanisms and properties of ion-transport in inorganic solid electrolytes. Energy Storage Mater 10:139–159. https://doi.org/10.1016/j.ensm.2017.08.015
Xiao Y, Miara LJ, Wang Y, Ceder G (2019) Computational screening of cathode coatings for solid-state batteries. Joule 2:1–24. https://doi.org/10.1016/j.joule.2019.02.006
Han X, Gong Y, Fu K et al (2016) Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat Mater 16:572–579. https://doi.org/10.1038/nmat4821
Sharafi A, Meyer HM, Nanda J, Wolfenstine J, Sakamoto J (2016) Characterizing the Li–Li7La3Zr2O12 interface stability and kinetics as a function of temperature and current density. J Power Sources 302:135–139. https://doi.org/10.1016/j.jpowsour.2015.10.053
Cheng EJ, Sharafi A, Sakamoto J (2017) Intergranular Li metal propagation through polycrystalline Li6.25Al0.25La3Zr2O12 ceramic electrolyte. Electrochim Acta 223:85–91. https://doi.org/10.1016/j.electacta.2016.12.018
Yu S, Schmidt RD, Garcia-Mendez R, Herbert E, Dudney NJ, Wolfenstine JB, Sakamoto J, Siegel DJ (2016) Elastic properties of the solid electrolyte Li7La3Zr2O12 (LLZO). Chem Mater 28:197–206. https://doi.org/10.1021/acs.chemmater.5b03854
Wang C, Li X, Zhao Y et al (2019) Manipulating interfacial nanostructure to achieve high-performance all-solid-state lithium-ion batteries. Small Methods 1900261:1900261. https://doi.org/10.1002/smtd.201900261
Bachman JC, Muy S, Grimaud A et al (2016) Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem Rev 116:140–162. https://doi.org/10.1021/acs.chemrev.5b00563
Meier K, Laino T, Curioni A (2014) Solid-state electrolytes: Revealing the mechanisms of Li-ion conduction in tetragonal and cubic LLZO by first-principles calculations. J Phys Chem C 118:6668–6679. https://doi.org/10.1021/jp5002463
Augustyn V, McDowell MT, Vojvodic A (2018) Toward an atomistic understanding of solid-state electrochemical interfaces for energy storage. Joule 2:2189–2193. https://doi.org/10.1016/j.joule.2018.10.014
Janek J, Zeier WG (2016) A solid future for battery development. Nat Energy 1:16141. https://doi.org/10.1038/nenergy.2016.141
Hao F, Han F, Liang Y, Wang C, Yao Y (2018) Architectural design and fabrication approaches for solid-state batteries. MRS Bull 43:775–781. https://doi.org/10.1557/mrs.2018.211
Kato Y, Hori S, Saito T et al (2016) High-power all-solid-state batteries using sulfide superionic conductors. Nat Energy 1:16030. https://doi.org/10.1038/nenergy.2016.30
van den Broek J, Afyon S, Rupp JLM (2016) Interface-engineered all-solid-state Li-ion batteries based on garnet-type fast Li+ conductors. Adv Energy Mater 6:1600736. https://doi.org/10.1002/aenm.201600736
Han F, Yue J, Chen C et al (2018) Interphase engineering enabled all-ceramic lithium battery. Joule 2:497–508. https://doi.org/10.1016/j.joule.2018.02.007
Sakuda A, Takeuchi T, Kobayashi H (2016) Electrode morphology in all-solid-state lithium secondary batteries consisting of LiNi1/3Co1/3Mn1/3O2 and Li2S-P2S5 solid electrolytes. Solid State Ionics 285:112–117. https://doi.org/10.1016/j.ssi.2015.09.010
Sakuda A, Hayashi A, Tatsumisago M (2017) Recent progress on interface formation in all-solid-state batteries. Curr Opin Electrochem 6:108–114. https://doi.org/10.1016/j.coelec.2017.10.008
Sakuda A, Hayashi A, Tatsumisago M (2013) Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery. Sci Rep 3:2261. https://doi.org/10.1038/srep02261
Machida N, Kashiwagi J, Naito M, Shigematsu T (2012) Electrochemical properties of all-solid-state batteries with ZrO2-coated LiNi1/3Mn1/3Co1/3O2 as cathode materials. Solid State Ionics 225:354–358. https://doi.org/10.1016/j.ssi.2011.11.026
Okada K, Machida N, Naito M, Shigematsu T, Ito S, Fujiki S, Nakano M, Aihara Y (2014) Preparation and electrochemical properties of LiAlO2-coated Li(Ni1/3Mn1/3Co1/3)O2 for all-solid-state batteries. Solid State Ionics 255:120–127. https://doi.org/10.1016/j.ssi.2013.12.019
Kitaura H, Hayashi A, Tadanaga K, Tatsumisago M (2010) Electrochemical performance of all-solid-state lithium secondary batteries with Li–Ni–Co–Mn oxide positive electrodes. Electrochim Acta 55:8821–8828. https://doi.org/10.1016/j.electacta.2010.07.066
Ohta N, Takada K, Sakaguchi I, Zhang L, Ma R, Fukuda K, Osada M, Sasaki T (2007) LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries. Electrochem Commun 9:1486–1490. https://doi.org/10.1016/j.elecom.2007.02.008
Sun S, Xia Q, Liu J et al (2019) Self-standing oxygen-deficient α-MoO3−x nanoflake arrays as 3D cathode for advanced all-solid-state thin film lithium batteries. J Mater 5:229–236. https://doi.org/10.1016/j.jmat.2019.01.001
Xu S, McOwen DW, Zhang L et al (2018) All-in-one lithium-sulfur battery enabled by a porous-dense-porous garnet architecture. Energy Storage Mater 15:458–464. https://doi.org/10.1016/j.ensm.2018.08.009
Gong Y, Fu K, Xu S et al (2018) Lithium-ion conductive ceramic textile: a new architecture for flexible solid-state lithium metal batteries. Mater Today 21:594–601. https://doi.org/10.1016/j.mattod.2018.01.001
Minke C, Kunz U, Turek T (2017) Carbon felt and carbon fiber—a techno-economic assessment of felt electrodes for redox flow battery applications. J Power Sources 342:116–124. https://doi.org/10.1016/j.jpowsour.2016.12.039
Hakimian A, Kamarthi S, Erbis S, Abraham KM, Cullinane TP, Isaacs JA (2015) Economic analysis of CNT lithium-ion battery manufacturing. Environ Sci Nano 2:463–476. https://doi.org/10.1039/c5en00078e
Acknowledgements
Funding was provided by the Research Corporation for Science Advancement (Scialog: Advanced Energy Storage) and Lyda Hill Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Spencer, M.A., Augustyn, V. Free-standing transition metal oxide electrode architectures for electrochemical energy storage. J Mater Sci 54, 13045–13069 (2019). https://doi.org/10.1007/s10853-019-03823-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03823-y