Abstract
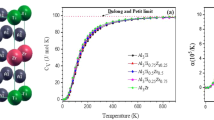
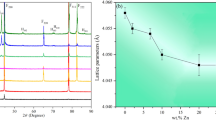
Hexagonal binary intermetallics A5B3 has a unique A6 octahedra chain structure, providing space for interstitial chemical engineering the physical, mechanical, electrical, and chemical properties without change in the basic structure of crystal. Because of the engineering importance of Zr–Sn alloy, here, we investigate the influence of 24 interstitial alloying elements X (X = B, C, N, O, Al, Si, P, S, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Nb, and Sn) on stability and properties of hexagonal Zr5Sn3 via first-principles calculations. A general trend is that the additional element with small atom size and high electronegativity is favorable as interstitials in Zr5Sn3. The calculated formation enthalpy and the elastic constants suggest that these Zr5Sn3X structures are thermodynamically and mechanically stable. The calculated phonon spectra indicate that Zr5Sn3X structures are dynamically stable except X = V, Cr, Mn, Zn, and Nb. We show that their electronic structures including bonding characters have strong correlation with the stability and mechanical properties. With strong covalent bonds, Zr5Sn3B has the highest Young’s modulus, bulk modulus, shear modulus, Debye temperature, and microhardness. The addition of alloying elements decreases the anisotropy except X = O, Sc, Ti, V and Nb. All the additive elements increase the specific heat capacity of Zr5Sn3. Our results could be helpful in designing and improve the performance of Zr–Sn alloy on demand.










Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wei J, Frankel P, Polatidis E, Blat M, Ambard A, Comstock RJ, Hallstadius L, Hudson D, Smith GDW, Grovener CRM, Klaus M, Cottis RA, Lyon S, Preuss M (2013) The effect of Sn on autoclave corrosion performance and corrosion mechanisms in Zr–Sn–Nb alloys. Acta Mater 61:4200–4214
Zhang R, Jiang B, Pang C, Dai X, Sun Y, Liao W, Wang Q, Dong C (2017) New low-Sn Zr cladding alloys with excellent autoclave corrosion resistance and high strength. Metals 7:144
McPherson DJ, Hansen M (1953) The system zirconium–tin. Trans ASM 45:915–933
Carpenter GJ, Ibrahim EF, Watters JF (1981) The aging response of zirconium–tin alloys. J Nucl Mater 102:280–291
Arias D, Roberti L (1983) The solubility of tin in α and β zirconium below 1000 °C. J Nucl Mater 118:143–149
Baykov VI, Perez RJ, Korzhavyi PA, Sundman B, Johansson B (2006) Structural stability of intermetallic phases in the Zr–Sn system. Scripta Mater 55(5):485–488
Perez RJ, Toffolon-Masclet C, Joubert JM, Sundman B (2008) The Zr–Sn binary system: New experimental results and thermodynamic assessment. Calphad 32(3):593–601
Krishna KM, Prakash DL, Timár G, Fitzner A, Srivastava D, Saibaba N, da Fonseca JQ, Dey GK, Preuss M (2016) The effect of loading direction and Sn alloying on the deformation modes of Zr: an in situ neutron diffraction study. Mater Sci Eng, A 650:497–509
Liu S, Zhan Y, Wu J, Wei X (2015) Insight into structural, mechanical, electronic and thermodynamic properties of intermetallic phases in Zr–Sn system from first-principles calculations. J Phys Chem Solids 86:177–185
Corbett JD, Garcia E, Guloy AM, Hurng WM, Kwon YU, Leon-Escamilla EA (1998) Widespread Interstitial Chemistry of Mn5Si3-Type and Related Phases: hidden Impurities and Opportunities. Chem Mater 10:2824–2836
Garcia E, Corbett JD (1990) Chemistry in the polar intermetallic host zirconium antimonide, Zr5Sb3 Fifteen interstitial compounds. Inorg Chem 29:3274–3282
Kwon YU, Corbett JD (1990) The zirconium–tin system, with particular attention to the Zr5Sn3-Zr5Sn4 region and Zr4Sn. Chem Mater 2:27–33
Kwon YU, Corbett JD (1992) Influence of oxygen on the stability of zirconium–tin (Zr4Sn). Chem Mater 4:187–190
Kwon YU, Corbett JD (1992) Chemistry in polar intermetallic compounds. The interstitial chemistry of zirconium–tin (Zr5Sn3). Chem Mater 4:1348–1355
Balińska A, Kordan V, Misztal R, Pavlyuk V (2015) Electrochemical and thermal insertion of lithium and magnesium into Zr5Sn3. J Solid State Electrochem 19:2481–2490
Blöchl PE (1994) Projector augmented-wave method. Phys Rev B 50:17953–17979
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59:1758–1777
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54:11169–11186
Kresse G, Furthmüller J (1996) Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6:15–50
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13:5188–5192
Monkhorst HJ, Pack JD (1989) High-precision sampling for Brillouin-zone integration in metals. Phys Rev B 40:3616–3621
Togo A. Phonopy. http://phonopy.sourceforge.net/
Togo A, Oba F, Tanaka I (2008) First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys Rev B 78:134106
Birch F (1947) Finite elastic strain of cubic crystals. Phys Rev B 71:809–824
Blanco MA, Francisco E, Luana V (2004) GIBBS: isothermal-isobaric thermodynamics of solids from energy curves using a quasi-harmonic Debye model. Comput Phys Commun 158:57–72
Wang SQ, Ye HQ (2003) Ab initio elastic constants for the lonsdaleite phases of C, Si and Ge. J Phys: Condens Matter 15:5307–5314
Wu ZJ, Zhao EJ, Xiang HP, Hao XF, Liu XJ, Meng J (2007) Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys Rev B 76:054115-1–054115-15
Voigt W (1928) Lehrbuch de Kristallphysik: Teubner-Leipzig. Macmillan, New York
Reuss A (1929) Berechnug der Flieβgrenze von Mischkristallen auf Grund der Plastizifäfsbedingung für Einkristalle. Angew Z Math Mech 9:49–58
Hill R (1952) The elastic behaviour of a crystalline aggregate. Proc Phys Soc Lond. A 65:349–354
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Novotny H, Auer-Welsbach H, Bruss J, Kohl A (1959) Ein Beitrag zur M5Si3-Struktur (D88-Typ). Monatsh Chem 90:15–23
Schubert K, Meissner HG, Raman A, Rossteutscher W (1964) Einige Strukturdaten metallischer Phasen. Naturewiss 51:287
Meschel SV, Kleppa OJ (1998) Standard enthalpies of formation of some 3d, 4d and 5d transition-metal stannides by direct synthesis calorimetry. Thermochim Acta 314:205–212
Yang J, Long J, Yang L, Li D (2013) First-principles investigations of the physical properties of binary uranium silicide alloys. J Nucl Mater 443:195–199
Haines J, Leger JM, Bocquillon G (2001) Synthesis and design of superhard materials. Annu Rev Mater Res 31:1–23
Chen XQ, Niu H, Li D, Li Y (2011) Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics 19:1275–1281
Tian Y, Xu B, Zhao Z (2012) Microscopic theory of hardness and design of novel superhard crystals. Int J Refr Met Hard Mater 33:93–106
Pauling L (1960) The nature of the chemical bond and the structure of molecules and crystals: an introduction to modern structural chemistry. Cornell University Press, New York
Pugh SF (1954) Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos Mag 45:823–843
Ravindran P, Fast L, Korzhavyi PA, Johansson B, Wills J, Eriksson O (1998) Density functional theory for calculation of elastic properties of orthorhombic crystals: application to TiSi2. J Appl Phys 84:4891–4904
Compton WD, Gschneidner KA Jr, Hutchings MT, Rabin H, Tosi MP (1964) Physical properties and interrelationships of metallic and semimetallic elements. In: Seitz F, Turnbull D (eds) Solid state physics: advanced in research and applications, vol 16. Academic Press, New York, p 321
Eshelby DJ (1954) Distortion of a crystal by point imperfections. J Appl Phys 25:255–261
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Grant Nos. 11464001, 51531009 and 51661003), the Guangxi Natural Science Foundation (2014GXNSFAA118308, 2016GXNSFBA380166).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, H., Cao, Y., Liu, K. et al. Stability and physical properties tuning via interstitials chemical engineering of Zr5Sn3: a first-principles study. J Mater Sci 54, 10284–10296 (2019). https://doi.org/10.1007/s10853-019-03622-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03622-5