Abstract
Numerical analysis of the heat balance at the flash event during flash sintering of granular ceramic nanoparticles was performed assuming continuum solid state as well as simultaneous surface softening/liquid formation and current percolation through the nanoparticle contacts. Assuming inter-particle radiations in the specimen volume, the electric Joule heat generated at the nanoparticle contacts partially lost by radiation from the specimen external surfaces. Considering the thermal effects due to rapid heating rate and free-molecular heat conduction regime, high-temperature gradients between the nanoparticle surfaces and the surrounding gas were developed. The attractive capillary forces, induced by the particle surface softening/liquid at the percolation threshold, lead to rapid rearrangement and densification of the nanoparticles. The excess Joule heat, already at the flash event, suffices the excess internal heat that is necessary for partial or full melting. Particle surface softening/liquid formation is a transient process, hence followed by crystallization immediate after the nanoparticle rearrangement. Thermal runaway is associated with local surface softening/melting and its solidification.




Similar content being viewed by others
References
Cologna M, Rashkova B, Raj R (2010) Flash sintering of nanograin zirconia in < 5 s at 850 C. J Am Ceram Soc 93:3556–3559
Cologna M, Francis JSC, Raj R (2011) Field assisted and flash sintering of alumina and its relationship to conductivity and MgO-doping. J Eur Ceram Soc 31:2827–2837
Lebrun JM, Raj R (2014) A first report of photoemission in experiments related to flash sintering. J Am Ceram Soc 97:2427–2430
McLaren C, Heffner W, Tessarollo R, Raj R, Jain H (2015) Electric field-induced softening of alkali silicate glasses. A Phys Lett 107:184101. Supplementary material at http://dx.doi.org/10.1063/1.4934945
Naik K, Jha SK, Raj R (2016) Correlation between conductivity, electroluminescence and flash sintering. Scr Mater 118:1–4
McLaren C, Roling B, Raj R, Jain H (2017) Mechanism of electric field-induced softening (EFIS) of alkali silicate glasses. J Non Cryst Solids 471:384–395
Biesuz M, Luchi P, Quaranta A, Martucci A, Sglavo VM (2017) Photoemission during flash sintering: an interpretation based on thermal radiation. J Eur Ceram Soc 37:3125–3130
Downs JA, Sglavo VM (2013) Electric field assisted sintering of cubic zirconia at 390 C. J Am Ceram Soc 96:1342–1344
Dong Y, Chen IW (2015) Predicting the onset of flash sintering. J Am Ceram Soc 98:2333–2335
Biesuz M, Luchi P, Quaranta A, Sglavo VM (2016) Theoretical and phenomenological analogies between flash sintering and dielectric breakdown in α-alumina. J Appl Phys 120:145107
Du Y, Stevenson AJ, Vernat D, Diaz M, Marinha D (2016) Estimating Joule heating and ionic conductivity during flash sintering of 8YSZ. J Eur Ceram Soc 36:749–759
Raj R (2016) Analysis of the power density at the onset of flash sintering. J Am Ceram Soc 99:3226–3232
Chaim R (2017) Particle surface softening as universal behaviour during flash sintering of oxide nano-powders. Materials 10:179
Todd RI, Zapata-Solvas E, Bonilla RS, Sneddon T, Wilshaw PR (2015) Electrical characteristics of flash sintering: thermal runaway of Joule heating. J Eur Ceram Soc 35:1865–1877
Zhang Y, Jung J, Luo J (2015) Thermal runaway, flash sintering and asymmetrical microstructural development of ZnO and ZnO–Bi2O3 under direct currents. Acta Mater 94:87–100
Pereira da Silva JG, Al-Qureshi HA, Keil F, Janssen R (2016) A dynamic bifurcation criterion for thermal runaway during the flash sintering of ceramics. J Eur Ceram Soc 36:1261–1267
Biesuz M, Sglavo VM (2016) Flash sintering of alumina: effect of different operating conditions on densification. J Eur Ceram Soc 36:2535–2542
Shomrat N, Baltianski S, Dor E, Tsur Y (2017) The influence of doping on flash sintering conditions in SrTi1-xFexO3-δ. J Eur Ceram Soc 37:179–188
Jiang T, Wang Z, Zhang J, Hao X, Rooney D, Liu Y, Sun W, Qiao J, Sun K (2015) Understanding the flash sintering of rare-earth-doped Ceria for solid oxide fuel cell. J Am Ceram Soc 98:1717–1723
Muccillo R, Kleitz M, Muccillo ENS (2011) Flash grain welding in yttria-stabilized zirconia. J Eur Ceram Soc 31:1517–1521
Raj R (2012) Joule heating during flash-sintering. J Eur Ceram Soc 32:2293–2301
Ji W, Parker B, Falco S, Zhang JY, Fu ZY, Todd RI (2017) Ultra-fast firing: effect of heating rate on sintering of 3YSZ, with and without an electric field. J Eur Ceram Soc 37:2547–2551
Bykov YV, Egorov SV, Eremeev AG, Kholoptsev VV, Rybakov KI, Sorokin AA (2015) Flash microwave sintering transparent Yb (LaY)2O3 ceramics. J Am Ceram Soc 98:3518–3524
Narayan J (2013) Grain growth model for electric-assisted processing and flash sintering of materials. Scr Mater 68:785–788
Chaim R (2016) Liquid film capillary mechanism for densification of ceramic powders during flash sintering. Materials 9:280
Corapcioglu G, Gulgun MA, Kisslinger K, Sturm S, Jha SK, Raj R (2016) Microstructural and microchemistry of flash sintered K0.5Na0.5NbO3. J Ceram Soc Japan 124:321–328
Grasso S, Sakka Y, Rendtorff N, Hu C, Maizza G, Borodianska H, Vasylkiv O (2011) Modeling of the temperature distribution of flash sintered zirconia. J Ceram Soc Japan 119:144–146
Terauds K, Lebrun JM, Lee HH, Jeon TY, Lee SH, Je JH, Raj R (2015) Electroluminescence and the measurement of temperature during stage III of flash sintering experiments. J Eur Ceram Soc 35:3195–3199
Park J, Chen IW (2013) In situ thermometry measuring temperature flashes exceeding 1700 C in 8 mol% Y2O3-stabilized zirconia under constant-voltage heating. J Am Ceram Soc 96:697–700
Lebrun JM, Jha SK, McCormack SJ, Kriven WM, Raj R (2016) Broadening of diffraction peak widths and temperature nonuniformity during flash experiments. J Am Ceram Soc 99:3429–3434
Pereira da Silva JG, Lebrun JM, Al-Qureshi HA, Janssen R, Raj R (2015) Temperature distribution during flash sintering of 8% yttria-stabilized zirconia. J Am Ceram Soc 98:3525–3528
Dong Y, Chen IW (2016) Thermal runaway in mold-assisted flash sintering. J Am Ceram Soc 99:2889–2894
Holland TB, Anselmi-Tamburini U, Quach DV, Tran TB, Mukherjee AK (2012) Effects of local Joule heating during the field assisted sintering of ionic ceramics. J Eur Ceram Soc 32:3667–3674
Chaim R, Weibel A, Chevallier G, Estournès C (2017) Flash sintering of dielectric nanoparticles as a percolation phenomenon through a softened film. J Appl Phys 121:145103
Yoshida H, Uehashi A, Tokunaga T, Sasaki K, Yamamoto T (2016) Formation of grain boundary second phase in BaTiO3 polycrystal under a high DC electric field at elevated temperatures. J Ceram Soc Jpn 124:388–392
Chen K, Jiao Y, Zhao Y, Gao Y, Zhang X, An L (2017) Non-contact electric field-enhanced abnormal grain growth in (K0.5Na0.5) NbO3 ceramics. Ceram Int 43:12343–12347
Du B, Gucci F, Porwal H, Grasso S, Mahajan A, Reece MJ (2017) Flash spark plasma sintering of magnesium silicide stannide with improved thermoelectric properties. J Mater Chem C 5:1514–1521
Kocjan A, Logar M, Shen Z (2017) The agglomeration, coalescence and sliding of nanoparticles, leading to the rapid sintering of zirconia nanoceramics. Sci Rep 7:2541
Liu F, Daun KJ, Snelling DR, Smallwood GJ (2006) Heat conduction from a spherical nano-particle: status of modeling heat conduction in laser-induced incandescence. Appl Phys B 83:355–382
Yoshida H, Morita K, Kim BN, Sakka Y, Yamamoto T (2016) Reduction in sintering temperature for flash-sintering of yttria by nickel cation-doping. Acta Mater 106:344–352
Merabia S, Shenogin S, Joly L, Keblinski P, Barrat JL (2009) Heat transfer from nanoparticles: a corresponding state analysis. PNAS 106:15113–15118
Merabia S, Keblinski P, Joly L, Lewis LJ, Barrat JL (2009) Critical heat flux around strongly heated nanoparticles. Phys Rev E 79:021404
Zhang Y, Luo J (2015) Promoting the flash sintering of ZnO in reduced atmospheres to achieve nearly full densities at furnace temperatures < 120 C. Scr Mater 106:26–29
Olevsky EA, Rolfing SM, Maximenko AL (2016) Flash (ultra-rapid) spark-plasma sintering of silicon carbide. Sci Rep 6:33408
Smith WF, Hashemi J (2000) Foundations of materials science and engineering. McGraw Hill, Boston, p 616
Muccillo R, Muccillo ENS (2013) An experimental setup for shrinkage evaluation during electric field-assisted flash sintering: application to yttria-stabilized zirconia. J Eur Ceram Soc 33:515–520
Michelsen HA (2003) Understanding and predicting the thermal response of laser-induced incandescence from carbonaceous particles. J Chem Phys 118:7012–7045
Fu H, Cohen RE (2000) Polarization rotation mechanism for ultrahigh electromechanical response in single-crystal piezoelectrics. Nature 403:281–283
Johari GP (2013) Effects of electric field on the entropy, viscosity, relaxation time, and glass-formation. J Chem Phys 138:154503
Biesuz M, Abate WD, Sglavo VM (2017) Porcelain stoneware consolidation by flash sintering. J Am Ceram Soc 101:71–81
Majidi H, van Benthem K (2015) Consolidation of partially stabilized ZrO2 in the presence of a noncontacting electric field. Phys Rev Lett 114:195503
Chaim R, Chevallier G, Weibel A, Estournès C (2018) Grain growth during spark plasma and flash sintering of ceramic nanoparticles: a review. J Mater Sci 53:3087–3105. https://doi.org/10.1007/s10853-017-1761-7
Schwesig D, Schierning G, Theissmann R, Stein N, Petermann N, Wiggers H, Schmechel R, Wolf DE (2011) From nanoparticles to nanocrystalline bulk: percolation effects in field assisted sintering of silicon nanoparticles. Nanotechnology 22:135601
Marder R, Estournès C, Chevallier G, Chaim R (2014) Plasma in spark plasma sintering of ceramic particle compacts. Scr Mater 82:57–60
Yadav D, Raj R (2017) Two unique measurements related to flash experiments with yttria-stabilized zirconia. J Am Ceram Soc 100:5374–5378
Karakuscu A, Cologna M, Yarotski D, Won J, Francis JSC, Raj R, Uberuaga BP (2012) Defect structure of flash-sintered strontium titanate. J Am Ceram Soc 95:2531–2536
Gouyet JF (1996) Physics and fractal structures. Masson, New York
Kirshenbaum AD, Cahill JA (1960) The density of liquid aluminium oxide. J Inorg Nucl Chem 14:283–287
Chase MW (1998) NIST-JANAF thermochemical tables. J Phys Chem Ref Data Monogr 9:1–1951
Acknowledgements
R. Chaim acknowledges the warm and kind hospitality of the colleagues from CIRIMAT during his sabbatical stay in Toulouse, where this paper was prepared. We thank Dr. Rachel Marder for kindly providing the Fig. 4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Appendix
Appendix
Thermal heat
The internal heat was calculated using the following equation:
While neglecting the change in the green density up to the flash temperature, which fairly is correct [56]. The temperature-dependent specific heat of solid alumina was used, up to its melting point.
When liquid forms, most probably at the nanoparticle contacts, the change in the internal heat was calculated according to the following equation [47]:
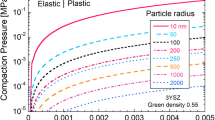
where xmelt is the volume fraction of the melt, and indices s and l refer to solid and liquid, respectively. Since current percolation is associated with the percolation phenomenon, the volume fraction of the melt is 0.247 at the invasive percolation threshold [57]. This value was used to calculate the heat capacity of the specimen when liquid forms.
For heat/energy balance, we added the enthalpy of fusion at the percolation threshold (i.e., for fusion of 0.247 volume fraction of the mass) to the internal heat versus temperature (see the shaded area in Fig. 2).
The internal heat in Eq. (9) was calculated using the finite differences approximation described elsewhere [18]:
where the interval between two consecutive temperatures and their corresponding time interval Δt were determined from the heating rate during the experiment [2].
The following temperature-dependent functions were used:
-
(a)
Temperature dependencies of the density (g cm−3) of solid Al2O3 and its melt (liquid) [58]:
$$ \rho_{\text{s}} = 3.9899 - 12 \times 10^{ - 5} \cdot T\left( {^\circ {\text{C}}} \right) $$(12)$$ \rho_{\text{l}} = 5.3243 - 11.27 \times 10^{ - 4} \cdot T\left( {^\circ {\text{C}}} \right) $$(13) -
(b)
Temperature dependence of the specific heat of solid and liquid Al2O3 (J mol−1 K−1) [59]:
$$ c_{\text{p}}^{\text{solid}} = 102.43 + 38.75 \cdot \theta - 15.91 \cdot \theta^{2} + 2.63 \cdot \theta^{3} - \frac{3.0075}{{\theta^{2} }} $$(14)in the temperature range 298–2327 K
$$ c_{\text{p}}^{\text{liquid}} = 192.46 + 9.52 \times 10^{ - 8} \cdot \theta - 2.85 \times 10^{ - 8} \cdot \theta^{2} + 2.93 \times 10^{ - 9} \cdot \theta^{3} - \frac{{5.59 \times 10^{ - 8} }}{{\theta^{2} }} $$(15)in the temperature range 2327–4000 K
$$ {\text{Where}}\quad \theta = \frac{{T\left( {\text{K}} \right)}}{1000} $$
Joule heat
The accumulated Joule heat was calculated using the following equation:
The temperature dependence of the electric resistivity of pure Al2O3 is strongly affected by the impurity content. Therefore, published data about flash sintering of alumina was used together with the specimen dimensions and the flash process parameters [2]. The average electric conductivity of the present alumina is therefore:
and the electric conductivity of alumina melt is [25]:
The Joule heat prior to any melt was calculated using the solid phase properties, both as dense, as well as a granular (percolative) system [34]. However, at the current percolation threshold, when continuous liquid forms, the specimen properties of a percolating media (with liquid volume fraction of 0.247) were used [34]:
Rights and permissions
About this article
Cite this article
Chaim, R., Estournès, C. On thermal runaway and local endothermic/exothermic reactions during flash sintering of ceramic nanoparticles. J Mater Sci 53, 6378–6389 (2018). https://doi.org/10.1007/s10853-018-2040-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2040-y