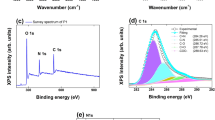
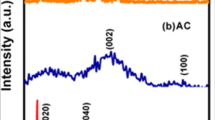
Abstract
In this study, activated carbon was derived from polypyrrole (PPY) using a K2CO3 activating agent with varying mass ratios of the activating agent to PPY polymer (AA:PP), for the optimization of the hierarchical pore structure necessary for improved electrochemical performance. The textural study of the as-synthesized samples (AC-PPY) displayed an increase in the specific surface area (SSA) and pore volume with increase in the amount of the activating agent up to a threshold for AA:PP of 6:1. The increase in the SSA was due to the presence of hierarchical pores in the material structure for efficient ion penetration. Initial half-cell electrochemical tests performed on the different activated carbon samples with varying SSA revealed superior charge storage capability for the 6:1 sample in both negative and positive operating potentials. The highest current response value was obtained from the signatory EDLC-type cyclic voltammogram, along with the longest discharge time from the chronopotentiometry plot as a result of the lowest ion diffusion length for successful fast ion transport reported from the impedance spectroscopy analysis. A full symmetric device (AC-PPY-6) assembled from the best material using KNO3 neutral electrolyte yielded a specific capacitance of 140 F g−1, 12.4 Wh kg−1 energy density at 0.5 A g−1 gravimetric current. An energy density of 7.12 Wh kg−1 was still maintained at a specific current of 2 A g−1. Interestingly, after the ageing test to ascertain device stability, the device energy density increased back to 12.2 Wh kg−1 as a result of the creation of additional active pores within the nanostructured material for charge storage via voltage holding tests which also led to the enhancement in specific capacitance to 137.5 F g−1 at 2 A g−1. A 99.0% capacitance retention was recorded even after 10000 cycles at a moderate specific current of 2 A g−1. A substantial approach was used to elucidate the degradation phenomena from the device self-discharge profile, which showcased the device retaining up to 70% of its operating potential after 80 h (> 3 days) on open circuit. The results obtained demonstrate the potential of adopting the AC-PPY material in potential device for energy storage purposes.




Similar content being viewed by others
References
Simon P, Gogotsi Y, Dunn B (2014) Where do batteries end and supercapacitors begin? Science 80(343):1210–1211
Lukatskaya MR, Dunn B, Gogotsi Y (2016) Multidimensional materials and device architectures for future hybrid energy storage. Nat Commun 7:12647
Chabi S, Peng C, Hu D, Zhu Y (2014) Ideal three-dimensional electrode structures for electrochemical energy storage. Adv Mater 26:2440–2445
Béguin F, Presser V, Balducci A, Frackowiak E (2014) Carbons and electrolytes for advanced supercapacitors. Adv Mater 26:2219–2251, 2283
Pandolfo AG, Hollenkamp AF (2006) Carbon properties and their role in supercapacitors. J Power Sources 157:11–27
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7:845–854
Simon P, Gogotsi Y (2013) Capacitive energy storage in nanostructured carbon-electrolyte systems. Acc Chem Res 46:1094–1103
Nishihara H, Kyotani T (2012) Templated nanocarbons for energy storage. Adv Mater 24:4473–4498
Yu C, Fan J, Tian B et al (2002) High-yield synthesis of periodic mesoporous silica rods and their replication to mesoporous carbon rods. Adv Mater 14:1742–1745
Meng W, Chen W, Zhao L, Huang Y, Zhu M, Huang Y, Fu Y, Geng F, Yu J, Chen X, Zhi C (2014) Porous Fe3O4/carbon composite electrode material prepared from metal-organic framework template and effect of temperature on its capacitance. Nano Energy 8:133–140
Kyotani T, Ma Z, Tomita A (2003) Template synthesis of novel porous carbons using various types of zeolites. Carbon 41:1451–1459
Basavalingu B, Calderon Moreno JM, Byrappa K et al (2001) Decomposition of silicon carbide in the presence of organic compounds under hydrothermal conditions. Carbon 39:1763–1766
Lillo-Rodenas MA, Cazorla-Amoros D, Linares-Solano A et al (2003) Understanding chemical reactions between carbons and NaOH and KOH: an insight into the chemical activation mechanism. Carbon 41:267–275
Raymundo-Pinero E, Azais P, Cacciaguerra T et al (2005) KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organisation. Carbon 43:786–795
Wang T, Tan S, Liang C (2009) Preparation and characterization of activated carbon from wood via microwave-induced ZnCl2 activation. Carbon 47:1880–1883
Liu Q-S, Zheng T, Wang P, Guo L (2010) Preparation and characterization of activated carbon from bamboo by microwave-induced phosphoric acid activation. Ind Crops Prod 31:233–238
Wang J, Kaskel S (2012) KOH activation of carbon-based materials for energy storage. J Mater Chem 22:23710
Wei L, Sevilla M, Fuertes AB et al (2011) Hydrothermal carbonization of abundant renewable natural organic chemicals for high-performance supercapacitor electrodes. Adv Energy Mater 1:356–361
Qie L, Chen W, Xu H et al (2013) Synthesis of functionalized 3D hierarchical porous carbon for high-performance supercapacitors. Energy Environ Sci 6:2497
Sevilla M, Mokaya R, Fuertes AB (2011) Ultrahigh surface area polypyrrole-based carbons with superior performance for hydrogen storage. Energy Environ Sci 4:2930–2936
Bello A, Manyala N, Barzegar F, Khaleed AA, Momodu DY, Dangebegnon JK (2016) Renewable pine cone biomass derived carbon materials for supercapacitor application. RSC Adv 6:1800–1809
Momodu D, Madito M, Barzegar F, Bello A, Khaleed AA, Olaniyan O, Dangbegnon J, Manyala N (2016) Activated carbon derived from tree bark biomass with promising material properties for supercapacitors. J Solid State Electrochem 21:859–872
Sevilla M, Fuertes AB (2016) A green approach to high-performance supercapacitor electrodes: the chemical activation of hydrochar with potassium bicarbonate. Chemsuschem 9:1880–1888
Snook GA, Kao P, Best AS (2011) Conducting-polymer-based supercapacitor devices and electrodes. J Power Sources 196:1–12
Huang Y, Li H, Wang Z, Zhu M, Pei Z, Xie Q, Huang Y, Zhi C (2016) Nanostructured polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 22:422–438
Huang Y, Tao J, Meng W, Zhu M, Huang Y, Fu Y, Gao Y, Zhi C (2015) Super-high rate stretchable polypyrrole-based supercapacitors with excellent cycling stability. Nano Energy 11:518–525
Yang P, Mai W (2014) Flexible solid-state electrochemical supercapacitors. Nano Energy 8:274–290
Bello A, Barzegar F, Madito MJ et al (2017) Floating of PPY derived carbon based symmetric supercapacitor in alkaline electrolyte. ECS Trans 6:3–5
Bello A, Barzegar F, Madito MJ, Momodu DY, Khaleed AA, Mashikhwa TM, Dangbegnon JK, Manyala N (2016) Stability studies of polypyrrole-derived carbon based symmetric supercapacitor via potentiostatic floating test. Electrochim Acta 213:107–114
Yao L, Yang G, Han P, Tang Z, Yang J (2016) Three-dimensional beehive-like hierarchical porous polyacrylonitrile-based carbons as a high performance supercapacitor electrodes. J Power Sources 315:209–217
Mao Y, Duan H, Xu B et al (2012) Lithium storage in nitrogen-rich mesoporous carbon materials. Energy Environ Sci 5:7950
Sadezky A, Muckenhuber H, Grothe H, Grothe H, Niesnner R, Poschl U (2005) Raman microspectroscopy of soot and related carbonaceous materials: spectral analysis and structural information. Carbon 43:1731–1742
Zhang H, Zhang L, Chen J, Su H, Liu F, Yang W (2016) One-step synthesis of hierarchically porous carbons for high-performance electric double layer supercapacitors. J Power Sources 315:120–126
Momodu DYY, Barzegar F, Abdulhakeem B, Dangbegnon J, Masikhwa T, Madito M, Manyala N (2015) Simonkolleite-graphene foam composites and their superior electrochemical performance. Electrochim Acta 151:591–598
Hu S, Zhang S, Pan N, Lo Hsieh Y (2014) High energy density supercapacitors from lignin derived submicron activated carbon fibers in aqueous electrolytes. J Power Sources 270:106–112
Peng C, Lang J, Xu S, Wang X (2014) Oxygen-enriched activated carbons from pomelo peel in high energy density supercapacitors. RSC Adv 4:54662–54667
Cherusseri J, Kar KK (2016) Hierarchical carbon nanopetal/polypyrrole nanocomposite electrodes with brush-like architecture for supercapacitors. Phys Chem Chem Phys 18:8587–8597
Chang Y, Han G, Chang Y, Xiao Y, Hou W, Zhou W (2017) Flexible and compressible electrochemical capacitors based on polypyrrole/carbon fibers integrated into sponge. J Alloys Compd 708:1206–1215
Shivakumara S, Kishore B, Penki TR, Munichandraiah N (2014) Symmetric supercapacitor based on partially exfoliated and reduced graphite oxide in neutral aqueous electrolyte. Solid State Commun 199:26–32
Bello A, Barzegar F, Momodu D, Dangbegnon J, Taghizadeh F, Manyala N (2015) Symmetric supercapacitors based on porous 3D interconnected carbon framework. Electrochim Acta 151:386–392
He X, Li R, Qiu J et al (2012) Synthesis of mesoporous carbons for supercapacitors from coal tar pitch by coupling microwave-assisted KOH activation with a MgO template. Carbon 50:4911–4921
Hao P, Zhao Z, Tian J et al (2014) Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 6:12120–12129
Wang Q, Yan J, Xiao Y et al (2013) Interconnected porous and nitrogen-doped carbon network for supercapacitors with high rate capability and energy density. Electrochim Acta 114:165–172
Andreas HA (2015) Self-discharge in electrochemical capacitors: a perspective article. J Electrochem Soc 162:A5047–A5053
Oickle AM (2013) A systematic study of self-discharge mechanisms in carbon-based, aqueous electrolyte electrochemical capacitors. Ph.D. dissertation, Chemistry Department, Dalhousie University
Reddy RN, Reddy RG (2006) Porous structured vanadium oxide electrode material for electrochemical capacitors. J Power Sources 156:700–704
Lao ZJ, Konstantinov K, Tournaire Y et al (2006) Synthesis of vanadium pentoxide powders with enhanced surface-area for electrochemical capacitors. J Power Sources 162:1451–1454
Acknowledgements
This work is based on the research supported by the South African Research Chairs Initiative of the Department of Science and Technology, Republic of South Africa, and National Research Foundation of South Africa (Grant No. 61056). Any opinion, finding, conclusion or recommendation expressed in this material is that of the author(s), and the NRF does not accept any liability in this regard. B. S. Moyo will like to acknowledge the SARChI Chair in Carbon for funding her Masters’ Degree project. D. Momodu will like to acknowledge financial support from the National Research Foundation (NRF) for his postdoctoral study. Finally, the authors would like to specially thank Dr. Farshad Barzegar of the Electrical and Electronics Engineering Department for his inputs through the invaluable and fruitful discussions which contributed to the final preparation of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moyo, B., Momodu, D., Fasakin, O. et al. Electrochemical analysis of nanoporous carbons derived from activation of polypyrrole for stable supercapacitors. J Mater Sci 53, 5229–5241 (2018). https://doi.org/10.1007/s10853-017-1911-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1911-y