Abstract
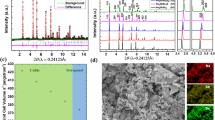
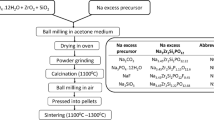
Exploration of advanced solid electrolytes is a highly relevant research topic for all-solid-state batteries and sensors. One of the effective ways to improve the ion transport is cation substitution. In this work, single-phase Na3P1−x Sb x Se4 polycrystalline compounds are synthesized via solid-state reaction method. The impact of Sb substitution on the chemical structure is revealed by X-ray powder diffraction and Raman spectroscopy. Sb substitution enlarges the unit cell and thus increases the ionic conductivity of Na3P1−x Sb x Se4. Na3SbSe4 as a fully substituted compound achieves the lowest activation energy value of 0.19 eV and the highest ionic conductivity value of 3.7 mS/cm, one of the best values among sulfide solid electrolytes. Together with a low grain-boundary resistance, a single Na+ transference number, and high thermal stability, Na3SbSe4 is a very promising solid electrolyte for all-solid-state sodium batteries.







Similar content being viewed by others
References
Larcher D, Tarascon JM (2015) Towards greener and more sustainable batteries for electrical energy storage. Nat Chem 7:19–29
Kundu D, Talaie E, Duffort V, Nazar LF (2015) The emerging chemistry of sodium ion batteries for electrochemical energy storage. Angew Chem Int Ed 54:3431–3448
Wen Z, Hu Y, Wu X, Han J, Gu Z (2013) Main challenges for high performance NAS battery: materials and interfaces. Adv Funct Mater 23:1005–1018
Pan H, Hu YS, Chen L (2013) Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ Sci 6:2338–2360
Bachman JC, Muy S, Grimaud A, Chang HH, Pour N, Lux SF, Paschos O, Maglia F, Lupart S, Lamp P, Giordano L, Shao-Horn Y (2016) Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem Rev 116:140–162
Kim JG, Son B, Mukherjee S, Schuppert N, Bates A, Kwon O, Choi MJ, Chung HY, Park S (2015) A review of lithium and non-lithium based solid state batteries. J Power Sources 282:299–322
Kalhoff J, Eshetu GG, Bresser D, Passerini S (2015) Safer electrolytes for lithium-ion batteries: state of the art and perspectives. Chemsuschem 8:2154–2175
Jung YS, Oh DY, Nam YJ, Park KH (2015) Issues and challenges for bulk-type all-solid-state rechargeable lithium batteries using sulfide solid electrolytes. Isr J Chem 55:472–485
Zhang L, Yang K, Dong J, Lu L (2015) Recent developments in thio-LISICON solid electrolytes. J Yanshan Univ 39:95–106
Yao X, Huang B, Yin J, Peng G, Huang Z, Gao C, Liu D, Xu X (2016) All-solid-state lithium batteries with inorganic solid electrolytes: review of fundamental science. Chin Phys B 25:018802
Vignarooban K, Kushagra R, Elango A, Badami P, Mellander BE, Xu X, Tucker TG, Nam C, Kannan AM (2016) Current trends and future challenges of electrolytes for sodium-ion batteries. Int J Hydrog Energy 41:2829–2846
Sun C, Liu J, Gong Y, Wilkinson DP, Zhang J (2017) Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 33:363–386
Tatsumisago M, Nagao M, Hayashi A (2013) Recent development of sulfide solid electrolytes and interfacial modification for all-solid-state rechargeable lithium batteries. J Asian Ceram Soc 1:17–25
Takada K (2013) Progress and prospective of solid-state lithium batteries. Acta Mater 61:759–770
Hayashi A, Sakuda A, Tatsumisago M (2016) Development of sulfide solid electrolytes and interface formation processes for bulk-type all-solid-state Li and Na batteries. Front Energy Res 4:25
Lin Z, Liang C (2015) Lithium–sulfur batteries: from liquid to solid cells. J Mater Chem A 3:936–958
Slater MD, Kim D, Lee E, Johnson CS (2013) Sodium-ion batteries. Adv Funct Mater 23:947–958
Palomares V, Casas-Cabanas M, Castillo-Martínez E, Han MH, Rojo T (2013) Update on Na-based battery materials: a growing research path. Energy Environ Sci 6:2312–2337
Hayashi A, Noi K, Sakuda A, Tatsumisago M (2012) Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries. Nat Commun 3:856
Hayashi A, Noi K, Tanibata N, Nagao M, Tatsumisago M (2014) High sodium ion conductivity of glass–ceramic electrolytes with cubic Na3PS4. J Power Sources 258:420–423
Tanibata N, Noi K, Hayashi A, Tatsumisago M (2014) Preparation and characterization of highly sodium ion conducting Na3PS4–Na4SiS4 solid electrolytes. RSC Adv 4:17120–17123
Berbano SS, Seo I, Bischoff CM, Schuller KE, Martin SW (2012) Formation and structure of Na2S + P2S5 amorphous materials prepared by melt-quenching and mechanical milling. J Noncryst Solids 358:93–98
Jha PK, Pandey OP, Singh K (2014) Crystallization and glass transition kinetics of Na2S–P2S5-based super-ionic glasses. Part Sci Technol 33:166–171
Noi K, Hayashi A, Tatsumisago M (2014) Structure and properties of the Na2S–P2S5 glasses and glass–ceramics prepared by mechanical milling. J Power Sources 269:260–265
Tanibata N, Noi K, Hayashi A, Kitamura N, Idemoto Y, Tatsumisago M (2014) X-ray crystal structure analysis of sodium-ion conductivity in 94 Na3PS4·6 Na4SiS4 glass-ceramic electrolytes. Chem Electro Chem 1:1130–1132
Hibi Y, Tanibata N, Hayashi A, Tatsumisago M (2015) Preparation of sodium ion conducting Na3PS4–NaI glasses by a mechanochemical technique. Solid State Ion 270:6–9
Zhu Z, Chu IH, Deng Z, Ong SP (2015) Role of Na+ interstitials and dopants in enhancing the Na+ conductivity of the cubic Na3PS4 superionic conductor. Chem Mater 27:8318–8325
Chu IH, Kompella CS, Nguyen H, Zhu Z, Hy S, Deng Z, Meng YS, Ong SP (2016) Room-temperature all-solid-state rechargeable sodium-ion batteries with a Cl-doped Na3PS4 superionic conductor. Sci Rep 6:33733
Klerk NJJ, Wagemaker M (2016) Diffusion mechanism of the sodium-ion solid electrolyte Na3PS4 and potential improvements of halogen doping. Chem Mater 28:3122–3130
Yu C, Ganapathy S, Klerk NJJ, Eck ERH, Wagemaker M (2016) Na-ion dynamics in tetragonal and cubic Na3PS4, a Na-ion conductor for solid state Na-ion batteries. J Mater Chem A 4:15095–15105
Wenzel S, Leichtweiss T, Weber DA, Sann J, Zeier WG, Janek J (2016) Interfacial reactivity benchmarking of the sodium ion conductors Na3PS4 and sodium beta-alumina for protected sodium metal anodes and sodium all-solid-state batteries. ACS Appl Mater Interfaces 8:28216–28224
Bo SH, Wang Y, Ceder G (2016) Structural and Na-ion conduction characteristics of Na3PSxSe4−x. J Mater Chem A 4:9044–9053
Pompe C, Pfitzner A (2012) Na3SbSe3: synthesis, crystal structure determination, raman spectroscopy, and ionic conductivity. Z Anorg Allg Chem 638:2158–2162
Kim SK, Mao A, Sen S, Kim S (2014) Fast Na-ion conduction in a chalcogenide glass–ceramic in the ternary system Na2Se–Ga2Se3–GeSe2. Chem Mater 26:5695–5699
Richards WD, Tsujimura T, Miara LJ, Wang Y, Kim JC, Ong SP, Uechi I, Suzuki N, Ceder G (2016) Design and synthesis of the superionic conductor Na10SnP2S12. Nat Commun 7:11009
Kandagal VS, Bharadwaj MD, Waghmare UV (2015) Theoretical prediction of a highly conducting solid electrolyte for sodium batteries: Na10GeP2S12. J Mater Chem A 3:12992–12999
Yu Z, Shang SL, Seo JH, Wang D, Luo X, Huang Q, Chen S, Lu J, Li X, Liu ZK, Wang D (2017) Exceptionally high ionic conductivity in Na3P0.62As0.38S4 with improved moisture stability for solid-state sodium-ion batteries. Adv Mater 29:1605561
Bo S-H, Wang Y, Kim JC, Richards WD, Ceder G (2016) Computational and experimental investigations of Na-ion conduction in cubic Na3PSe4. Chem Mater 28:252
Tian Y, Shi T, Richards WD, Li J, Bo S-H (2017) Compatibility issues between electrodes and electrolytes in solid-state batteries. Energ Environ Sci 10:1150
Zhang L, Yang K, Mi J, Lu L, Zhao L, Wang L, Li Y, Zeng H (2015) Na3PSe4: a novel chalcogenide solid electrolyte with high ionic conductivity. Adv Energy Mater 5:1501294
Zhang L, Zhang D, Yang K, Yan X, Wang L, Mi J, Xu B, Li Y (2016) Vacancy-contained tetragonal Na3SbS4 superionic conductor. Adv Sci 3:1600089
Trevey JE, Jung YS, Lee SH (2010) Preparation of Li2S–GeSe2–P2S5 electrolytes by a single step ball milling for all-solid-state lithium secondary batteries. J Power Sources 195:4984–4989
Kim J, Yoon Y, Eom M, Shin D (2012) Characterization of amorphous and crystalline Li2S–P2S5–P2Se5 solid electrolytes for all-solid-state lithium ion batteries. Solid State Ion 225:626–630
Liu Z, Tang Y, Wang Y, Huang F (2014) High performance Li2S–P2S5 solid electrolyte induced by selenide. J Power Sources 260:264–267
Yang K, Dong J, Zhang L, Li Y, Wang L, Stevenson J (2015) Dual doping: an effective method to enhance the electrochemical properties of Li10GeP2S12-based solid electrolytes. J Am Ceram Soc 98:3831–3835
Rodriguez-Carvajal J (1992) Recent advances in magnetic structure determination by neutron powder diffraction. Phys B 192:55–69
Hayashi A, Muramatsu H, Ohtomo T, Hama S, Tatsumisago M (2014) Improved chemical stability and cyclability in Li2S–P2S5–P2O5–ZnO composite electrolytes for all-solid-state rechargeable lithium batteries. J Alloys Compd 591:247–250
Wan H, Peng G, Yao X, Yang J, Cui P, Xu X (2016) Cu2ZnSnS4/graphene nanocomposites for ultrafast, long life all-solid-state lithium batteries using lithium metal anode. Energy Storage Mater 4:59–65
Acknowledgements
This work was supported by the National Science Foundation of China (51525205) and the Science Foundation of Hebei Education Department (ZD2016033).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, N., Yang, K., Zhang, L. et al. Improvement in ion transport in Na3PSe4–Na3SbSe4 by Sb substitution. J Mater Sci 53, 1987–1994 (2018). https://doi.org/10.1007/s10853-017-1618-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1618-0