Abstract
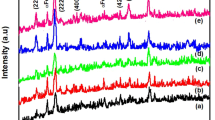
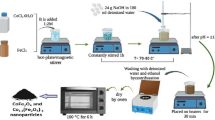
The report states that well-dispersed CoFe2O4 nanoparticles (NPs) with controllable morphology were prepared using an economical and facile one-pot thermal decomposition approach. Cobalt (II) acetylacetonate and Iron (III) acetylacetonate were employed as precursors instead of expensive and toxic pentacarbonyl. The transmission electron microscopy and powder X-ray diffraction investigation show that CoFe2O4 NPs possess cubic morphology, homogeneous size distribution and pure phase structure. Optical band gap was tuned from 1.147 to 0.92 eV and saturation magnetization (M s) increased from 53.91 to 84.01 emu/g for the as-prepared and annealed (700 °C) NPs. The coercivity (H c) enhanced from 1137 to 2109 Oe at room temperature, which is the highest value reported to date for CoFe2O4 NPs synthesized by thermal decomposition. All CoFe2O4 (as-prepared and annealed) NPs showed excellent ferromagnetism behaviour at room temperature. Raman studies of CoFe2O4 NPs confirm the redistribution of Co2+ from octahedral to tetrahedral site. The work demonstrates the great potential of CoFe2O4 NPs as a promising alternative for data storage device applications as well as for opto-magnetic devices.








Similar content being viewed by others
References
Ling D, Hyeon T (2013) Iron oxide nanoparticles: chemical design of biocompatible iron oxide nanoparticles for medical applications. Small 9:1450–1466
Jeong JM, Choi BG, Lee SC, Lee KG, Chang SJ, Han YK, Lee YB, Lee HU, Kwon S, Lee G, Lee CS, Huh YS (2013) Hierarchical hollow spheres of Fe2O3@polyaniline for lithium ion battery anodes. Adv Mater 25:6250–6255
Chopdekar RV, Suzuki Y (2006) Magnetoelectric coupling in epitaxial CoFe2O4 on BaTiO3. Appl Phys Lett 89:182506
Zeng H, Li J, Liu JP, Wang ZL, Sun SH (2002) Exchange-coupled nanocomposite magnets by nanoparticle self-assembly. Nature 420:395
Park J, An K, Hwang Y, Park JG, Noh HJ, Kim JY, Park JH, Hwang NM, Hyeon T (2004) Ultra-large-scale syntheses of monodispersenanocrystals. Nat Mater 3:891
Sun SH (2006) Recent advances in chemical synthesis, self-assembly, and applications of FePt nanoparticles. Adv Mater 18:393–403
Qu Y, Yang H, Yang N, Fan Y, Zhu H, Zou G (2006) The effect of reaction temperature on the particle size, structure and magnetic properties of coprecipitated CoFe2O4 nanoparticles. Mater Lett 60:3548–3552
Zhang Y, Yang Z, Yin D, Liu Y, Fei C, Xiong R, ShiJ Yan G (2010) Composition and magnetic properties of cobalt ferrite nano-particles prepared by the co-precipitation method. J Magn Magn Mater 322:3470–3475
Maaz K, Mumtaz A, Hasanain SK, Ceylan A (2007) Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J Magn Magn Mater 308:289–295
Naik SR, SalKer AV (2012) Change in the magnetostructural properties of rare earth doped cobalt ferrites relative to the magnetic anisotropy. J Mater Chem 22:2740–2750
Zan F, Ma Y, Ma Q, Xu Y, Dai Z, Zheng G, Wu M, Li G (2013) Magnetic and impedance properties of nanocomposite CoFe2O4/Co0.7Fe0.3 and single-phase CoFe2O4 prepared via a one-step hydrothermal synthesis. J Am Ceram Soc 96:3100–3107
Chinnasamy CN, Jeyadevan B, Shinoda K, TohjiK Djayaprawira DJ (2003) Unusually high coercivity and critical single-domain size of nearly monodispersed CoFe2O4 nanoparticles. Appl Phys Lett 83:2862
Eom Y, Abbas M, Noh HY, Kim CG (2016) Morphology-controlled synthesis of highly crystalline Fe3O4 and CoFe2O4 nanoparticles using a facile thermal decomposition method. Rsc Adv 6:15861–15867
Mordina B, Tiwari RK, Setua DK, Sharma A (2015) Superior elastomeric nanocomposites with electrospunnanofibers and nanoparticles of CoFe2O4 for magnetorheological applications. Rsc Adv 5:19091–19105
Ravindra AV, Padhan P, Prellier W (2012) Electronic structure and optical band gap of CoFe2O4 thin films. Appl Phys Lett 101:161902
Holinsworth BS, Mazumdar D, Sims H, Sun QC, Yurtisigi MK, Sarker SK, Gupta A, Butler WH, Musfeldt JL (2013) Chemical tuning of the optical band gap in spinel ferrites: CoFe2O4 versus NiFe2O4. Appl Phys Lett 103:082406
Abbas M, Rao BP, Islam MN, Kim KW, Naga SM, Takahashi M, Kim C (2014) Size-controlled high magnetization CoFe2O4 nanospheres and nanocubes using rapid one-pot sonochemical technique. Ceram Int 40:3269–3276
Abbas M, Rao BP, Kim C (2014) Shape and size-controlled synthesis of Ni Zn ferrite nanoparticles by two different routes. Mater Chem Phys 147:443–451
Ahn T, Kim JH, Yang HM, Lee JW, Kim JD (2012) Formation pathways of magnetite nanoparticles by Co-precipitation method. J Phys Chem C 116:6069–6076
Shirsath et al (2016) Switching of magnetic easy-axis using crystal orientation for large perpendicular coercivity in CoFe2O4 thin film. Sci Rep 6:30074
Rana AK, Kumar Y, Saxena N, Das R, Sen S, Shirage PM (2015) Studies on the control of ZnO nanostructures by wet chemical method and plausible mechanism. AIP Adv 5:097118
Kumar Y, Rana AK, Bhojane P, Pusty M, Bagwe V, Sen S, Shirage PM (2015) Controlling of ZnO nanostructures by solute concentration and its effect on growth, structural and optical properties. Mater Res Express 2:105017
Iqbal J, Rajpoot M, Jan T, Ahmad I (2014) Annealing induced enhancement in magnetic properties of Co0.5Zn0.5Fe2O4 nanoparticles. J Supercond Nov Magn 27:1743–1749
Wolska E, Riedel E, Wolski W (1992) The evidence of \( {\text{Cd}}^{2 + } {}_{x}{\text{Fe}}_{1 - x}^{3 + } \left[ {{\text{Ni}}_{1 - x}^{2 + } {\text{Fe}}_{1 + x}^{3 + } } \right]{\text{O}}_{4} \) cation distribution based on X-ray and mössbauerdata. Phys Stat Sol 132:K51–K56
Wu L, Olivier JP, David B, Wayne I, Alshakim N, Huiyuan Z, SenZ Shouheng S (2014) Monolayer assembly of ferrimagnetic Co x Fe3–x O4 nanocubes for magnetic recording. Nano Lett 14:3395–3399
Yang H, Ogawa T, Hasegawa D, Takahashi M (2008) Synthesis and magnetic properties of monodisperse magnetite nanocubes. J Appl Phys 103:07D526
Xia Y, Xoing Y, LimB Skrabalak SE (2009) Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics. Angew Chem Int Ed 48:60–103
Khurshid H, Li W, Chandra S, Phan MH, Hadjipanayis GC, Mukherjee P, Srikanth H (2013) Mechanism and controlled growth of shape and size variant core/shell FeO/Fe3O4 nanoparticles. Nanoscale 5:7942–7952
Zhou B, Zhang YW, Yu YJ, Liao CS, Yan CH (2003) Correlation between structure and intervalence charge-transfer transitions in nanocrystalline CoFe2−x M x O4 (M = Mn, Al, Sc) thin films. Phys Rev B 68:024426
Burns RG (1993) Mineralogical applications of crystal field theory. Cambridge University Press, Cambridge
Dileep K, Loukya B, Pachauri N, Gupta A, Datta R (2014) Probing optical band gaps at the nanoscale in NiFe2O4 and CoFe2O4 epitaxial films by high resolution electron energy loss spectroscopy. Appl Phys Lett 111:103505
Kumar CSSR (2012) Raman spectroscopy for nanomaterials characterization. Springer, Heidelberg
Chandramohan P, Srinivasan MP, Velmurugan S, Narasimhan SV (2011) Cation distribution and particle size effect on Raman spectrum of CoFe2O4.J. Solid State Chem 184:89–96
Yu T, Shen ZX, Shi Y, Ding J (2002) Cation migration and magnetic ordering in spinel CoFe2O4 powder: micro-Raman scattering study. J Phys 14:L613
Chamritski I, Burns G (2005) Infrared- and raman-active phonons of magnetite, maghemite, and hematite: a computer simulation and spectroscopic study. J Phys Chem B 109:4965–4968
Fan X, Guan J, Cao X, Wang W, Mou F (2010) Low-temperature synthesis, magnetic and microwave electromagnetic properties of substoichiometric spinel cobalt ferrite octahedra. Eur J Inorg Chem 3:419–426
Dunitz JD, Orgel LE (1957) Electronic properties of transition-metal oxides-II: cation distribution amongst octahedral and tetrahedral sites. J Phys Chem Solids 3:318–323
Ammar S, Helfen A, Jouini N, Fiévet F, Rosenman I, Villain F, MoliniéPh Danot M (2001) Magnetic properties of ultrafine cobalt ferriteparticles synthesized by hydrolysis in a polyol medium. J Mater Chem 11:186–192
Hanh N, Quy OK, Thuy NP, Thung LD (2003) Synthesis of cobalt ferrite nanocrystallites by the forced hydrolysis method and investigation of their magnetic properties. Physica B 327:382–384
Smit J, Wijn HPJ (1959) Ferrites. Wiley, New York, p 369
Kodama RH, Berkowitz AE (1996) Surface spin disorder in NiFe2O4 nanoparticles. Phys Rev Lett 77:394
Turtelli RS, Atif M, Mehmooda N, Kubel F, Biernacka K, Linert W, Grössingera R, Cz Kapusta, Sikora M (2012) Interplay between the cation distribution and production methods in cobalt ferrite. Mater Chem Phys 132:832–838
Sharma D, Khare N (2014) Tuning of optical bandgap and magnetization of CoFe2O4 thin films. App Phys Lett 105:032404
Fan X, Guan J, Cao X, Wang W, Mou F (2010) Low-temperature synthesis, magnetic and microwave electromagnetic properties of substoichiometric spinel cobalt ferrite octahedra. Eur J Inorg Chem 40:419–426
Yang W, Yu Y, Wang L, Yang C, Li H (2015) Controlled synthesis and assembly into anisotropic arrays of magnetic cobalt-substituted magnetite nanocubes. Nanoscale 7:2877–2882
Soundararajan D, Kim KH (2014) Synthesis of CoFe2O4 magnetic nanoparticles by thermal decomposition. J Magn 19:5–9
Xu ST, Ma YQ, Zheng GH, Dai ZX (2015) Simultaneous effects of surface spins: rarely large coercivity, high remanence magnetization and jumps in the hysteresis loops observed in CoFe2O4 nanoparticles. Nanoscale 7:6520–6526
Moya C, Morales MP, Batlle X, Labarta A (2015) Tuning the magnetic properties of Co-ferrite nanoparticles through the 1,2-hexadecanediol concentration in the reaction mixture. Phys Chem Chem Phys 17:13143–13149
Tegus O, Bruck E, Buschow KHJ, Boer FRD (2002) Transition-metal-based magnetic refrigerants for room-temperature applications. Nature 415:150
Franco AJ, Zapf V (2008) Temperature dependence of magnetic anisotropy in nanoparticles of Co x Fe(3−x)O4. J Magn Magn Mater 320:709–713
Bean C, Livingston J (1959) Superparamagnetism. J Appl Phys 30:1205
Kumar L, Kumar P, Kar M (2013) Cation distribution by Rietveld technique and magnetocrystalline anisotropy of Zn substituted nanocrystalline cobalt ferrite. J Alloys Compd 551:72–81
Tomita S, Jonsson PE, Akamatsu K, Nawafune H, Takayama H (2007) Controlled magnetic properties of Ni nanoparticles embedded in polyimide films. Phys Rev B 76:174432
Kurtan U, Topkaya R, Baykal A (2013) A sol–gel auto-combustion synthesis of PVP/CoFe2O4 Nano-composite and its magnetic characterization. Mater Res Bull 48:4889–4895
Vargas JM, Nunes WC, Socolovsky LM, Knobel M, Zanchet D (2005) Effect of dipolar interaction observed in iron-based nanoparticles. Phys Rev B 72:184428
Kneller EF, Luborsky FE (1963) Particle size dependence of coercivity and remanence of single domain particles. J Appl Phys 34:656–658
Guimaraes AP (2009) Nano-science and technology: principles of nanomagnetism. Springer, Berlin
Pal D, Mandal M, Chaudhuri A, Das B, Sarkar D, Mandal K (2010) Micelles induced high coercivity in single domain cobalt-ferrite nanoparticles. J Appl Phys 108:124317
Acknowledgements
This work was supported by the Department of Science and Technology, India, which awarded the prestigious ‘Ramanujan Fellowship’ (SR/S2/RJN-121/2012) to PMS, and CSIR research grant no. 03 (1349)/16/EMR-II was also awarded to PMS. PMS is greatly thankful to Dr. Pradeep Mathur, director, IIT Indore, for encouraging the research work and providing the necessary facilities. We express our gratitude to SIC, IIT Indore for providing the XRD characterization facility. We are also thankful to Dr. Chandrachure Mukherjee, Raja Ramanna Centre for Advanced Technology, Indore for providing the UV–Visible-NIR spectroscopy measurement facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, Y., Shirage, P.M. Highest coercivity and considerable saturation magnetization of CoFe2O4 nanoparticles with tunable band gap prepared by thermal decomposition approach. J Mater Sci 52, 4840–4851 (2017). https://doi.org/10.1007/s10853-016-0719-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0719-5