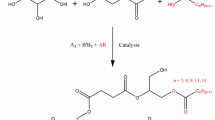
Abstract
The worldwide production of glycerin exceeds the demand from the chemical industry and new applications have been investigated these last years mainly in the area of additives. Here, water–soluble glycerin-based polyacrylates and polymethacrylates have been prepared by free-radical polymerization of solketal (meth)acrylate using various transfer agents derived from fatty chains. According to specific experimental conditions, linear functional oligomers were obtained. This versatile process allowed designing amphiphilic copolymers after the hydrolysis of acetal groups. These oligomers exhibit critical aggregation concentration values ranging from 5 to 15 × 10−6 mol.L−1 without any strong differences between polyacrylate and polymethacrylate series. In the case of glycerol-based polyacrylate derivatives, the observed monomodal populations can be ascribed to the low polydispersity indexes.





Similar content being viewed by others
References
Bagheri S, Julkapli NM, Yehye WA (2015) Catalytic conversion of biodiesel derived raw glycerol to value added products. Renew Sustain Energy Rev 41:113. doi:10.1016/j.rser.2014.08.031
Zhang H, Grinstaff MW (2014) Recent advances in glycerol polymers: chemistry and biomedical applications. Macromol Rapid Commun 35:1906. doi:10.1002/marc.201400389
Zhou CHC, Beltramini JN, Fan YX, Lu GQM (2008) Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem Soc Rev 37:527. doi:10.1039/b707343g
Mouloungui Z, Pelet S (2001) Study of the acyl transfer reaction: structure and properties of glycerol carbonate esters. Eur J Lipid Sci Technol 103:216. doi:10.1002/1438-9312(200104)103:4<216:aid-ejlt216>3.0.co;2-j
Besse V, Camara F, Mechin F et al (2015) How to explain low molar masses in PolyHydroxyUrethanes (PHUs). Eur Polymer J 71:1. doi:10.1016/j.eurpolymj.2015.07.020
Maisonneuve L, Lamarzelle O, Rix E, Grau E, Cramail H (2015) Isocyanate-free routes to polyurethanes and poly(hydroxy urethane)s. Chem Rev 115:12407. doi:10.1021/acs.chemrev.5b00355
Rokicki G, Rakoczy P, Parzuchowski P, Sobiecki M (2005) Hyperbranched aliphatic polyethers obtained from environmentally benign monomer: glycerol carbonate. Green Chem 7:529. doi:10.1039/b501597a
Guerin W, Helou M, Slawinski M, Brusson JM, Carpentier JF, Guillaume SM (2014) Macromolecular engineering via ring-opening polymerization (3): trimethylene carbonate block copolymers derived from glycerol. Polym Chem 5:1229. doi:10.1039/c3py00955f
Pham PD, Monge S, Lapinte V, Raoul Y, Robin JJ (2013) Various radical polymerizations of glycerol-based monomers. Eur J Lipid Sci Technol 115:28. doi:10.1002/ejlt.201200202
Nanda MR, Zhang YS, Yuan ZS, Qin WS, Ghaziaskar HS, Xu CB (2016) Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: a review. Renew Sustain Energy Rev 56:1022. doi:10.1016/j.rser.2015.12.008
Decker C, Moussa K (1990) A new class of highly reactive acrylic monomers. 1. Light-induced polymerization. makromolekulare Chemie-Rapid. Communications 11:159
Decker C, Moussa K (1991) A new class of highly reactive acrylic monomers. 2. light-induces copolymerization with difunctional oligomers. Makromolekulare Chemie 192:507–522
Moussa K, Decker C (1993) Light-induced polymerization of new highly reactive acrylic monomers. J Polym Sci Part A 31:2197. doi:10.1002/pola.1993.080310903
Giacomelli C, Borsali R (2008) ATRP of silylated glycerol monomethacrylate in organic medium for convenient synthesis of amphiphilic copolymers. Macromol Rapid Commun 29:573. doi:10.1002/marc.200700803
Rotta J, Pham PD, Lapinte V, Borsali R, Minatti E, Robin JJ (2014) Synthesis of amphiphilic polymers based on fatty acids and glycerol-derived monomers: a study of their self-assembly in water. Macromol Chem Phys 215:131
Ikkala O, ten Brinke G (2004) Hierarchical self-assembly in polymeric complexes: towards functional materials. Chem Commun. doi:10.1039/b403983a
Jenekhe SA, Chen XL (1999) Self-assembly of ordered microporous materials from rod-coil block copolymers. Science 283:372. doi:10.1126/science.283.5400.372
Liu GJ, Ding JF, Guo A, Herfort M, BazettJones D (1997) Potential skin layers for membranes with tunable nanochannels. Macromolecules 30:1851. doi:10.1021/ma961530b
Liu GJ, Qiao LJ, Guo A (1996) Diblock copolymer nanofibers. Macromolecules 29:5508. doi:10.1021/ma9604653
Rodriguez-Hernandez J, Checot F, Gnanou Y, Lecommandoux S (2005) Toward ‘smart’ nano-objects by self-assembly of block copolymers in solution. Prog Polym Sci 30:691. doi:10.1016/j.progpolymsci.2005.04.002
Yan XH, Liu FT, Li Z, Liu GJ (2001) Poly(acrylic acid)-lined nanotubes of poly(butyl methacrylate)-block-poly(2-cinnamoyloxyethyl methacrylate). Macromolecules 34:9112. doi:10.1021/ma0112927
Zhang LF, Eisenberg A (1996) Morphogenic effect of added ions on crew-cut aggregates of polystyrene-b-poly(acrylic acid) block copolymers in solutions. Macromolecules 29:8805. doi:10.1021/ma961376t
Lim H, Kassim A, Huang N, Yarmo MA (2009) Palm-based nonionic surfactants as emulsifiers for high internal phase emulsions. J Surfactants Deterg 12:355. doi:10.1007/s11743-009-1138-2
Bhatia SR, Mourchid A, Joanicot M (2001) Block copolymer assembly to control fluid rheology. Curr Opin Colloid Interface Sci 6:471. doi:10.1016/s1359-0294(01)00122-4
Hamley IW (2003) Nanotechnology with soft materials. Angewandte Chemie-Inter Edit 42:1692. doi:10.1002/ange.200200546
Renggli K, Baumann P, Langowska K, Onaca O, Bruns N, Meier W (2011) Selective and responsive nanoreactors. Adv Funct Mater 21:1241. doi:10.1002/adfm.201001563
Adams ML, Lavasanifar A, Kwon GS (2003) Amphiphilic block copolymers for drug delivery. J Pharm Sci 92:1343. doi:10.1002/jps.10397
Blanazs A, Armes SP, Ryan AJ (2009) Self-assembled block copolymer aggregates: from micelles to vesicles and their biological applications. Macromol Rapid Commun 30:267. doi:10.1002/marc.200800713
Miyata K, Christie RJ, Kataoka K (2011) Polymeric micelles for nano–scale drug delivery. React Funct Polym 71:227. doi:10.1016/j.reactfunctpolym.2010.10.009
Theato P, Zentel R, Schwarz S (2002) Synthesis of end-functionalized lipopolymers and their characterization with regard to polymer–supported lipid membranes. Macromol Biosci 2:387. doi:10.1002/1616-5195(200211)2:8<387:aid-mabi387>3.0.co;2-5
Giardi C, Lapinte V, Charnay C, Robin JJ (2009) Nonionic polyoxazoline surfactants based on renewable source: synthesis, surface and bulk properties. React Funct Polym 69:643. doi:10.1016/j.reactfunctpolym.2009.04.008
Stemmelen M, Travelet C, Lapinte V, Borsali R, Robin J-J (2013) Synthesis and self-assembly of amphiphilic polymers based on polyoxazoline and vegetable oil derivatives. Polym Chem 4:1445. doi:10.1039/c2py20840g
Travelet C, Stemmelen M, Lapinte V, Dubreuil F, Robin J-J, Borsali R (2013) Amphiphilic copolymers based on polyoxazoline and grape seed vegetable oil derivatives: self-assemblies and dynamic light scattering. J Nanopart Res. doi:10.1007/s11051-013-1626-1
Camara F, Caillol S, Boutevin B (2014) Free radical polymerization study of glycerin carbonate methacrylate for the synthesis of cyclic carbonate functionalized polymers. Eur Polymer J 61:133. doi:10.1016/j.eurpolymj.2014.10.001
Besse V, Camara F, Voirin C, Auvergne R, Caillol S, Boutevin B (2013) Synthesis and applications of unsaturated cyclocarbonates. Polym Chem 4:4545. doi:10.1039/c3py00343d
Loubat C, Boutevin B (2000) Analysis of the telomerization of methyl methacrylate by thioglycolic acid. application to determining the probabilities of structures of synthesized telomers. Macromol Chem Phys 201:2853. doi:10.1002/1521-3935(20001201)201:18<2853:aid-macp2853>3.0.co;2-h
Boyer C, Loubat C, Robin JJ, Boutevin B (2004) Synthesis of functionalized amine oligomers by free-radical telomerization of methyl methacrylate with a peculiar telogen: 2-aminoethanethiol hydrochloride. J Polym Sci Part A 42:5146. doi:10.1002/pola.20303
Loubat C, Boutevin B (2000) Telomerization of acrylic acid with thioglycolic acid: effect of the solvent on the C-T value. Polym Bull 44:569. doi:10.1007/s002890070080
Loubat C, Boutevin B (2001) Telomerization of acrylic acid with mercaptans: part 2. Kinetics of the synthesis of star–shaped macromolecules of acrylic acid. Polym Int 50:375. doi:10.1002/pi.638
Loubat C, Javidan A, Boutevin B (2000) Analysis of the telomerization of acrylic acid by mercaptans, 1: telomerization of acrylic acid by thioglycolic acid; Influence of the natural of the solvent on the value of the transfer constant and k(p)/k(te)1/2. Macromol Chem Phys 201:2845. doi:10.1002/1521-3935(20001201)201:18<2845:aid-macp2845>3.0.co;2-7
Aguiar J, Carpena P, Molina-Bolivar JA, Ruiz CC (2003) On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J Colloid Interface Sci 258:116. doi:10.1016/s0021-9797(02)00082-6
Du H, Yang X, Pang X, Zhai G (2014) The synthesis, self-assembling, and biocompatibility of a novel O-carboxymethyl chitosan cholate decorated with glycyrrhetinic acid. Carbohydr Polym 111:753. doi:10.1016/j.carbpol.2014.04.095
Amado E, Augsten C, Maeder K, Blume A, Kressler J (2006) Amphiphilic water soluble triblock copolymers based on poly(2,3-dihydroxypropyl methacrylate) and poly(propylene oxide): synthesis by atom transfer radical polymerization and micellization in aqueous solutions. Macromolecules 39:9486. doi:10.1021/ma060794n
Jansen J, Dias AA, Dorschu M, Coussens B (2003) Fast monomers: factors affecting the inherent reactivity of acrylate monomers in photoinitiated acrylate polymerization. Macromolecules 36:3861. doi:10.1021/ma021785r
Kaur B, D’Souza L, Slater LA et al (2011) Model random polyampholytes from nonpolar methacrylic esters. Macromolecules 44:3810. doi:10.1021/ma200357u
Rossi NAA, Zou Y, Scott MD, Kizhakkedathu JN (2008) RAFT synthesis of acrylic copolymers containing poly(ethylene glycol) and dioxolane functional groups: toward well-defined aldehyde containing copolymers for bioconjugation. Macromolecules 41:5272. doi:10.1021/ma800606k
Vo C-D, Cadman CJ, Donno R, Goos JACM, Tirelli N (2013) Combination of episulfide ring-opening polymerization with ATRP for the preparation of amphiphilic block copolymers. Macromol Rapid Commun 34:156. doi:10.1002/marc.201200636
Yang J, Zhang D, Jiang S, Yang J, Nie J (2010) Synthesis of Y–shaped poly(solketal acrylate)-containing block copolymers and study on the thermoresponsive behavior for micellar aggregates. J Colloid Interface Sci 352:405. doi:10.1016/j.jcis.2010.09.014
Wilhelm M, Zhao CL, Wang YC et al (1991) Polymer micelle formation. 3. Poly(styrene-ethylene oxide) block copolymer micelle formation in water: a fluorescence probe study. Macromolecules 24:1033. doi:10.1021/ma00005a010
Zhen Y, Wan SR, Liu YQ, Yan HS, Shi RF, Wang CH (2005) Atom transfer radical polymerization of solketal acrylate using cyclohexanone as the solvent. Macromol Chem Phys 206:607. doi:10.1002/macp.200400414
Whittaker MR, Urbani CN, Monteiro MJ (2008) Synthesis of linear and 4-arm star block copolymers of poly(methyl acrylate-b–solketal acrylate) by SET-LRP at 25 degrees C. J Polym Sci Part A 46:6346. doi:10.1002/pola.22946
Acknowledgement
The authors gratefully acknowledge ONIDOL for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pham, P.D., Monge, S., Lapinte, V. et al. Synthesis of surfactants by polymerization of glycerol (meth)acrylates with fatty acids derivatives as chain ends. J Mater Sci 52, 968–980 (2017). https://doi.org/10.1007/s10853-016-0392-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0392-8