Abstract
Flexible polyurethane foams (FPF) are polymer materials that have high flammability. Fyrol PNX (FPNX) and expandable graphite (EG), have been used to modify the properties of these materials. The aim of this study was to assess the possibility of improving the thermal stability and flame retardancy of FPF by the addition of FPNX and EG fillers. The prepared foams were characterised by their apparent density, hardness, flexibility, irreversible strain and linear flammability, as well as thermogravimetric analysis (TGA), dynamic mechanical analysis, Fourier transform infrared spectroscopy (FT-IR) and pyrolysis combustion flow calorimetry (PCFC) measurements. The apparent density, hardness, flexibility and irreversible strain results showed that the addition of graphite and phosphorous fillers to the FPF makes slight changes to the mechanical properties, which remain within the acceptable norms. It was also observed that reducing the amount of Fyrol PNX and replacing it with the same amount of EG allowed similar values of linear flammability to be obtained with a simultaneous increase in thermal stability, as shown in the TGA study and the PCFC test. Moreover, it was found that the modification of flexible polyurethane foam by the addition of a mixture of FPNX and EG fillers allows the best properties of this type of materials to be obtained. This result indicates that this type of modification could be an effective way to improve the thermal stability of FPF.
Similar content being viewed by others
Introduction
Polyurethanes (PUR) are polymer materials that have a wide range of applications in industry and households because of their numerous valuable properties and the ability to control many of their parameters during preparation. Flexible polyurethane foams (FPF) are widely used in many industries, including the furniture, construction and automotive fields. These materials are also commonly used in mattresses and upholstery foams. FPF have many advantages, such as a wide range of flexibility and hardness, good cushioning, high durability during use, good thermal and acoustic insulation properties, resistance to chemicals, low price and low density [1–3]. The main disadvantage of FPF is their high flammability. During the combustion of flexible polyurethane foam, a substantial quantity of toxic fumes is evolved. These toxic combustion products are mainly Carbon monoxide(CO), CO2, Hydrogen cyanide (HCN) and NO2. CO and HCN compounds are particularly dangerous. CO is dangerous for people at any concentration. In lower concentrations, it causes loss of movement coordination, and in higher concentrations, it may lead to sudden death. Hydrogen cyanide (HCN) is one of the most toxic gases. Only 0.011 vol.% causes death after 30–60 min, and 0.025 vol.% causes sudden death [4, 5]. In many applications (e.g., in vehicles and aircraft), all safety standards concerning the flammability and value of toxic substances that evolve during combustion must be met. For this reason, FPF are modified to reduce their flammability and smoke toxicity, while maintaining adequate mechanical properties. There are many types of modifications of FPF. The reduction of flammability is possible through the use of physical or chemical methods. In recent years, a popular method of modification has been the production of FPF with the addition of bromine and chlorine compounds. These methods provide an effective way to reduce the flammability of FPF, but bromine and chlorine fillers are halogen compounds that evolve dioxins, furans and other toxic substances during a fire. For this reason, these fillers have been banned in some countries (e.g., Germany). Restriction laws have also been imposed on the use of halogen compounds. For example, in February 2003 Restriction of Hazardous Substances Directive (RoHS) was adopted by the European Union. This directive restricts use of six hazardous substances including polybrominated biphenyls (PBB) and polybrominated diphenyl ether (PBDE) which are used as a flame retardants. Moreover, from 1989 exist Montreal Protocol on Substances that Deplete the Ozone Layer. The treaty is structured around several groups of halogenated hydrocarbons which plays a role in ozone depletion. All of these substances contains chlorine or bromine compounds. This protocol is designed to protect the ozone layer by phasing out the production of numerous substances containing these compounds. These restrictions have led to the search for new fillers that can reduce the flammability of polymers while remaining environmentally friendly and relatively safe for human health [6].
In these studies, phosphorous (Fyrol PNX) and expandable graphite (EG) fillers have been investigated. Materials from the Fyrol group are commonly used to increase the thermal resistance of FPF and other polymer materials [7–9]. They give relatively good results, but they are quite expensive. In recent years, EG has been the subject of studies related to lowering the flammability of polymer materials [10–16]. EG is a product of natural origin that has specific properties. It is formed through the special physical and chemical treatment of graphite ore. EG is used as an additive causing the increase of fire resistance of insulations, plastics and coatings. As a result of heating above 150–200 °C occurs the large volume expansion (about 250 times) of EG (it is formed expanded graphite), according to redox reaction between H2SO4 and graphite. The blowing gases are formed according to the reaction [10, 12, 14]:
Expanded graphite forms a tight membrane that protects material by preventing oxygen and flames from accessing the deeper layers of the material during combustion. Studies indicate that the addition of EG may favourably affect the flammability reduction of polyurethanes [10, 14]. The greatest advantage of this material is its low price, which great reduces expenses in industrial production. The effect of the addition of FPNX and EG on flammability and thermal and mechanical properties has been investigated.
Experimental
Materials
FPF were prepared by the one-step method. The polyol used to produce the FPF was Arcol Polyol 1108 from Bayer Materials Science. Toluene diisocyanate (TDI) from Zachem S.A. was used as an isocyanate. Water was used as a blowing agent. Four types of FPF were tested in this study. The first foam was pure flexible polyurethane foam (FPF), and the others were made with the addition of a phosphorous filler (FPF + FPNX), a graphite filler (FPF + EG) and a mixture of the two fillers (FPF + FPNX + EG). At the beginning of the production of the FPF, some part of the polyol was mixed with a specified quantity of the filler using a mechanical stirrer. Then, this mixture was mixed with the remainder of the polyol. The following steps remained the same for foams with and without fillers, following the one-step production method that is widely used in the production of FPF.
FPF are formed as a result of the chemical reactions between the components. The urethane linkage is formed as a result of the reaction between the isocyanate groups with the hydroxyl groups from polyol [14, 17]: 
The reaction between the water and the isocyanate leads to the production of carbon dioxide, which causes foaming of the mixture. The scheme of the foaming reaction is as follows:
The urea linkage is formed as a result of the reaction between amines (from reaction between water and isocyanate) and isocyanate groups: 
Previous publications include a wide range of quantities of the fillers in polyurethane foams, from 1 up to 40 wt% [1, 3, 10, 14]. Following a review of the literature and considering the limits in the preparation of the polyol mixture with fillers (a high quantity of filler produces a high viscosity of the mixture), it was decided to initially use the following amounts of fillers: FPF + FPNX—2.5 wt% of FPNX, FPF + EG—2.5 wt% of EG, FPF + FPNX + EG—1.25 wt% of FPNX and 1.25 wt% of EG. The phosphorous filler Fyrol PNX was obtained from ICL Industrial Products. EG with a particle size of ~0.18 mm and an expansion of ~250 was obtained from Sinograf (Poland).
Methods
Physical and mechanical measurements
The apparent density tests were carried out according to PN-77/C-05012.03. The samples were measured and weighed. The value of the apparent density was calculated as a mass/volume ratio. The hardness tests were carried out according to PN-EN ISO 3386-1:2000 using a Zwick machine. Cuboid foams with sizes of 100 × 100 × 50 mm were placed in handles and compressed. The hardness value was the force required to compress the foam to 40 % of its height. Flexibility tests were conducted according to PN C 04255:1997. Three samples of ~80 × 80 × 50 mm of each foam were measured. Irreversible strain tests were conducted according to PN-77/C-05012.10.
Thermogravimetric analysis (TGA)
TGA was used to analyse the thermal stability of the prepared foams. The TGA were performed using a TA Instruments TGA Q500. Samples of ~8 mg were heated to 600 °C at a heating rate of 10 °C/min in a nitrogen atmosphere.
Dynamic mechanical analysis (DMA)
DMA were carried out in a DMA Q800 from TA Instruments. Samples with dimensions of 10 × 10 × 5 mm were tested using a multi- frequency mode with a compression clamp. The samples were heated at a heating rate of 3 °C/min from −100 to 60 °C. The frequency was 5 Hz.
Fourier transform infrared spectroscopy (FT-IR)
FT-IR was performed using a Nicolet 6700 FT-IR. The samples were scanned 64 times with a resolution of 4 cm−1 over the wavenumbers range from 4000-400 cm−1. The spectral analyses were performed using an Omnic spectral analyser. On the basis of the FT-IR spectra of the analysed foams, the degree of phase separation (DPS) was determined by analysing the intensities of the carbonyl stretching vibrations of the free and hydrogen-bonded groups. A straight baseline was then drawn in the spectrum between 1760 and 1620 cm−1, and the carbonyl stretching zone was corrected by subtracting the baseline. To estimate the signal strengths, peak modelling of the infrared active carbonyl bands was carried out using the Gaussian curve-fitting method in the Omnic spectral analyser. The carbonyl absorption bands were deconvoluted with a number of iterations to determine the best absorbance fits. From the intensities of the characteristic absorbances (A), the degree of phase separation (DPS) could be obtained from Eq. (1), according to Pretsch et al. [18].
Flammability measurements
Linear flammability tests were carried out according to PN ISO 3795:1996. Samples were exposed to the action of a low energy flame. During this test, the time to flame extinguishment or time to burning of the whole foam was defined.
The Pyrolysis Combustion Flow Calorimeter (PCFC) tests were performed according to ASTM D7309-2007 (Determining Flammability Characteristics of Plastics and Other Solid Materials Using Microscale Combustion Calorimetry). The pyrolysis temperature range was from 75 to 750 °C, with a heating rate of 1 °C/s. The flow rate of O2/N2 was 20/80 cm3/min.
Results and discussion
Physical and mechanical properties
Table 1 presents the physical and mechanical properties of the examined foams. The addition of fillers into a flexible polyurethane foam caused an increase in its apparent density and hardness. The flexibility decreased in all foams containing fillers. The addition of FPNX to the flexible polyurethane foam raised the irreversible strain value higher than that of foam without any filler. The addition of EG did not change the value of the irreversible strain, and the addition of FPNX with EG caused only slight changes to this value. Summarizing, addition of fillers to the flexible polyurethane foam, led to deterioration in the physical and mechanical properties. It indicates, that presence of fillers in foams causes some inappropriate changes in the structure of flexible polyurethane foam. This may be related to the changes in foaming process, occurring as a result of the presence of fillers; addition of fillers to the flexible polyurethane foam, led to extending the rise time of tested foams (especially to the foam with FPNX addition). Further research are needed to verify these assumptions. However, these results remain within the acceptable norms.
TGA
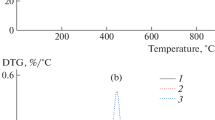
TGA was performed to assess the effect of the addition of phosphorous and graphite filler and a combination of the two fillers on the thermal stability of the flexible polyurethane foam. Figures 1 and 2 show thermograms of the research foams. Table 2 summarises the results of the selected parameters obtained from the TGA thermograms. The decomposition of polyurethanes occurs in two well-defined stages [19, 20]. The temperature corresponding to 5 % mass loss is considered to be the initial temperature of the sample’s decomposition process [21]. The value of 5 % mass loss decreased in the foam with the addition of FPNX in comparison to the value for FPF without fillers. Higher values of this temperature were observed in foams with the addition of EG and the combination of FPNX + EG. The temperature of 50 % mass loss corresponds to the temperature range of the decomposition of soft segments. The value of this temperature increased with the presence of each investigated filler. The largest increase was observed in the foam with added FPNX + EG. Tmax1 is defined as the temperature of the maximum rate of decomposition of rigid segments in polyurethane. The addition of FPNX reduced this value, but the addition of EG and FPNX + EG caused this value to increase higher than the value of FPF without fillers. Tmax2 corresponds to the temperature of the maximum rate of decomposition of soft segments of the polyurethane. The addition of any filler led to an increase in the value of Tmax2. The results obtained from TGA indicate that the thermal stability of the foams modified with the addition of research fillers is higher than that of foams without modification. The combination of both FPNX and EG fillers produced the best results for the thermal stability. This result is confirmed by the values of 5 % mass loss, 50 % mass loss, Tmax1 and Tmax2. This result indicates that this type of modification could be an effective way to improve the thermal stability of FPF. Furthermore, it should be noted that these changes are not large, and it is possible that higher contents of fillers should be examined to assess the influence of filler content on the properties of FPF. The value of the residue at 600 °C was almost two to three times higher than the value of the solid residue after the decomposition of the foams. The highest value of the residue occurred in the foam with EG (FPF + EG). This result is expected because EG has good thermal stability and its decomposition processes starts in temperatures above 2000 °C. Moreover, residue values after TGA indicate that there were a good dispersion of fillers in tested foams.
DMA
DMA is an useful technique to reveal some parameters of polymer materials (e.g., the glass transition temperature (T g), the energy dissipation and the stiffness). The measurement of the storage modulus (E′), the loss modulus (E″) and the loss tangent (tan δ) allows the determination of the glass transition temperature or the softening temperature. Table 3 presents the data obtained from DMA, and Fig. 3 presents the curves of the storage modulus of the tested foams.
The storage modulus describes the stiffness of the material and its ability to store energy [22]. The value of the storage modulus was higher in foams with the addition of fillers. The highest value of the storage modulus was determined for the foam with the addition of FPNX + EG. This result indicates that this foam had the best energy absorption of the tested foams. The loss modulus parameter describes a material’s ability to dissipate energy. Tan δ is the measurement of internal friction, and the curves of tan δ allow the T g values to be determined. Obtaining the glass transition temperature from the tan δ curves is the most common technique [22, 23]. The foam with added EG had the highest T g value.
FT-IR
FT-IR is a research technique that allows the chemical structure of a tested material to be analysed. In this study, the influence of the added fillers on the chemical structure of the tested foams was investigated. The typical bonds observed in polyurethanes were studied. Typical peaks observed in polyurethanes are discussed below. The peak at 3296.8 cm−1 is assigned to the N–H stretching vibrations [19, 23–27]. The peaks at 2971 and 2867 cm−1 are from the asymmetric and symmetric stretching vibrations of CH2, respectively [2, 19, 24]. The peak at ~2270 cm−1 is assigned to the N=C=O group [1, 2, 19, 28]. The peak at ~1717 cm−1 is assigned to the stretching vibrations of hydrogen-bonded C=O groups [17, 19, 24, 28–31]. The peak at ~1530 cm−1 is from amide II (urethane N–H bending + C–N stretching) [2, 25, 26]. The peak at 1085 cm−1 is assigned to the C–O–C stretching vibrations [24, 29, 30]. Figure 4 illustrates the FT-IR spectrum of the polyurethane foams investigated in this study. Typical peaks for polyurethanes are present.
Figure 5 presents the FT-IR spectra of all researched foams. The addition of filler to FPF caused only slight changes in the chemical structure of the FPF. Every peak that is characteristic of FPF is observed.
The introduction of EG led the degree of phase separation to increase, whereas the introduction of FPNX led it to decrease (Table 4). This result indicates that EG helps to form a greater number of hydrogen bonds, linking the carbonyl groups of the urethane and urea groupings with the N–H groups, but the introduction of FPNX causes the number to decrease. When both additives were introduced, the DPS of the obtained foam was the same as that of the foam in its initial state.
Flammability properties
Figure 6 presents the results of the linear flammability test. The addition of FPNX to the flexible polyurethane foam allows a lower value of the linear flammability to be obtained than the value for the foam without filler. EG did not improve the fire resistance of flexible polyurethane foam, but the combination of both fillers in foam allows the lowest value of the linear flammability to be obtained from all tested foams. This result means that the combination of FPNX and EG could be a good method to reduce the flammability of FPF.
The Pyrolysis Combustion Flow Calorimeter (PCFC) test is considered to be a useful tool for assessment of the fire behaviour of mg-sized samples, but it should be remembered that this type of test does not include some effects, such as dripping, swelling or the creation of an insulation barrier, which are present in large-scale tests [32–34]. Figure 7 presents the heat release rate (HRR) curves of the tested foams. As we can observe, the degradation of the tested foams occurs in two steps, as confirmed by TGA analysis. The first decomposition step (peak 1) can be considered a degradation product of the TDI compounds, and the second decomposition step (peak 2) may be associated with the decomposition of polyether [20]. The addition of research fillers to the FPF led to shift slightly to the right both of these peaks.
The HRR data, the time at which we observe the peak of the HRR (tHRR) and the temperature at which we can observe the peak of the HRR (THRR) were obtained from the PCFC, and they are presented in Table 5 (data relevant to the first peak in the graph) and Table 6 (data relevant to the second peak in the graph - the peak of the maximum HRR).
The results in Table 5 show that the addition of EG and FPNX + EG extended the time and increased the temperature needed to reach the first peak of the HRR. The time and temperature required to reach the first maximum of the HRR (respectively, tHRR1 and THRR1) were the longest for the foam containing FPNX + EG. Moreover, it should be noted that the value of the HRR of the first peak (HRR1) was lowest in the foam with FPNX + EG. The value of the HRR is also the value of the heat release capacity (HRC) in this case. This equivalence follows from the fact that the heating rate was 1 °C/s (HRC = HRR/heating rate) [20]. The heat release capacity is a measure of the real capacity of the material to undergo combustion. This value is considered to be the most important parameter obtained from the PCFC test and the best measurement of the fire hazard of a research material [20, 33]. These results means that the addition of FPNX + EG to FPF allows better thermal stability and more efficient combustion resistance to be obtained in the first stage of the decomposition of FPF. The results collected in Table 6 indicate that the addition of the EG or FPNX + EG filler leads to an increase in the HRRmax value. Only the FPNX addition cause the HRRmax value to become lower than that of the foam without any filler. However, any research filler allows the time and temperature required to reach the HRRmax peak to be increased (both of these parameters were the highest for the foam with FPNX + EG).
Conclusions
As a result of the thermal analysis and flammability measurements, it was found that the addition of FPNX + EG allows the best thermal stability and fire retardancy properties to be obtained. The foam with Fyrol PNX obtained better linear flammability and HRRmax value results than the foam with EG, but its thermal stability from the TGA measurement was worse than the value for the foam with EG. It was also observed that reducing the amount of Fyrol PNX and replacing it with the same amount of EG allows similar values of linear flammability to be achieved with a simultaneous increase in the thermal stability shown in the TGA study. Moreover, the addition of graphite and phosphorous filler to the FPF cause only slight changes in the chemical structure and mechanical properties, both of which remain within the acceptable norms. The PCFC measurements showed that the addition of FPNX + EG into FPF led to an extension of the time and an increase of the temperature of the maximum HRR. This method of modification could provide an effective way to reduce the flammability of FPF. However, it should be noted that changes in the properties of flexible polyurethane foam modified with 2.5 wt% addition of the research fillers were not huge, and it is possible that higher contents of fillers should be examined in future research, to assess the influence of the filler content on the properties of FPF.
References
Duquesne S, Delobel R, Le Bras M, Camino G (2002) Polym Degrad Stab 77:333
Sultan M, Bhatti HN, Zuber M, Bhatti IA, Sheikh MA (2011) Carbohydr Polym 86:928
Mahfuz H, Rangari VK, Islam MS, Jeelani S (2004) Composites 35:453
Sawicki T (2003) Bezp Pr 7–8:43
Iwko J (2007) Bezpieczeństwo użytkowania tworzyw sztucznych w aspekcie ich palności. Mech 10
Janowska G Przygocki W, Wlochowicz A (2007) Palność polimerów i materiałów polimerowych. WNT, Warsaw
Luda MP, Bracco P, Costa L, Levchik SV (2004) Polym Degrad Stab 83:215
Luda MP, Nada P, Costa L, Bracco P, Levchik SV (2004). Polym Degrad Stab 86:33
Levchik S, Piotrowski A, Weil E, Yao Q (2005) Polym Degrad Stab 88:57
Modesti M, Lorenzeti A, Simioni F, Camino G (2002) Polym Degrad Stab 77:195
Kuan C-F, Yen W-H, Chen C-H, Yuen S-M, Kuan H-C, Chiang C-L (2008) Polym Degrad Stab 93:1357
Modesti M, Lorenzetti A (2002) Polym Degrad Stab 78:167
Shiha Y-F, Wang Y-T, Jeng R-J, Wei K-M (2004) Polym Degrad Stab 86:339
Modesti M, Lorenzetti A (2003) Eur Polym J 39:263
Zhu H, Zhu Q, Li J, Tao K, Xue L, Yan Q (2011) Polym Degrad Stab 96:183
Fukushima K, Murariu M, Camino G, Dubois P (2010) Polym Degrad Stab 95:1063
Elwell MJ, Ryan AJ, Grunbauer HJM, Van Lieshout HC (1996) Polym 37:1353
Pretsch T, Jakob I, Muller W (2009) Polym Degrad Stab 94:61
Abdel Hakim AA, Nassar M, Emam A, Sultan M (2011) Mater Chem Phys 129:301
Krämer RH, Zammarano M, Linteris GT, Gedde UW, Gilman JW (2010) Polym Degrad Stab 95:1115
Ge X-G, Wang D-Y, Wang C, Qu M-H, Wang J-S, Zhao C-S, Jing X-K, Wang Y-Z (2007) Eur Polym J 43:2882
Leszczynska A, Pielichowski K (2008) J Therm Anal Calorim 93:677
Rivera-Armenta JL, Heinze Th, Mendoza-Martínez AM (2004) Eur Polym J 40:2803
Rogulska M, Kultys A, Podkościelny W (2007) Eur Polym J 43:1402
McCarthy SJ, Meijs GF, Mitchell N, Gunatillake PA, Heath G, Brandwood A, Schindhelm K (1997) Biomater 18:1387
Irusta L, Fernandez-Berridi MJ (2000) Polym 41:3297
Chen T-K, Tien Y-I, Wei K-H (2000) Polym 41:1345
Duquesne S, Le Bras M, Bourbigot S, Delobel R, Camino G, Eling B, Lindsay C, Roels T (2001) Polym Degrad Stab 74:493
Yilgor I, Yilgor E, Guclu Guler I, Ward TC, Wilkes GL (2006) Polym 47:4105
Mishra AK, Chattopadhyay DK, Sreedhar B, Raju KVSN (2006) Prog Org Coat 55:231
Modesti M, Zanella L, Lorenzetti A, Bertani R, Gleria M (2005) Polym Degrad Stab 87:287
Lyon RE, Walters RN (2004) J Anal Appl Pyrol 71:27
Schartel B, Pawlowski KH, Lyon RE (2007) Thermochim Acta 462:1
Lu H, Wilkie CA (2010) Polym Degrad Stab 95:2388
Acknowledgements
Financial support for this research was provided by the National Center of Research and Development as a grant No N R15 0024 06/2009. Special thanks are addressed to Zachem S.A. in Bydgoszcz and Institute of Natural Fibers and Medicinal Plants in Poznan for help in the realization of these studies.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wolska, A., Goździkiewicz, M. & Ryszkowska, J. Thermal and mechanical behaviour of flexible polyurethane foams modified with graphite and phosphorous fillers. J Mater Sci 47, 5627–5634 (2012). https://doi.org/10.1007/s10853-012-6433-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-012-6433-z