Abstract
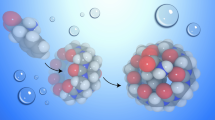
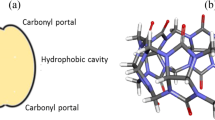
Molecular dynamics simulations were carried out to investigate the structure and dynamics of the 1:1 and 1:2 inclusion complexes formed by nor-Seco-cucurbit[10]uril (ns-CB[10]) with 1-adamantanmethylammonium in water. Two and three orientational isomers were considered for 1:1 and 1:2 complexes, respectively. These isomers are identified by the orientation/position of the ammonium group in the guest relative to the flexible and rigid carbonyl portals of the ns-CB[10] host. Results demonstrate that the inclusion of one guest molecule within one cavity in the host induces similar conformational changes in both the occupied and empty cavities. The average structure for each complex shows that the guest molecule is shifted closer to the side that lacks the CH2-bridge in the host, and that the ammonium group of the guest interacts with the oxygens of the host’s portals via ion–dipole interactions with the hydrophobic part of the guest molecule resides in the cavity of the host. Furthermore, the 1:1 complexes are found to interconvert over the time of the simulation. This observation is not found for 1:2 complexes. Finally, MM–PBSA calculations show that 1:2 complexes are significantly more stable than their 1:1 counterparts while two orientations of the 1:2 complexes are more stable than the third.








Similar content being viewed by others
References
Lagona, J., Mukhopadhyay, P., Chakrabarti, S., Isaacs, L.: The cucurbit[n]uril family. Angew. Chem. Int. Ed. 44, 4844–4870 (2005)
Masson, E., Ling, X., Joseph, R., Kyeremeh-Mensah, L., Lu, X.: Cucurbituril chemistry: a tale of supramolecular success. RSC Adv. 2, 1213–1247 (2012)
Isaacs, L.: Stimuli responsive systems constructed using cucurbit[n]uril-type molecular containers. Acc. Chem. Res. 47, 2052–2062 (2014)
Ko, Y.H., Hwang, I., Lee, D.W., Kim, K.: Ultrastable host-guest complexes and their applications. Isr. J. Chem. 51, 506–514 (2011)
Nau, W.M., Florea, M., Assaf, K.I.: Deep inside cucurbiturils: physical properties and volumes of their inner cavity determine the hydrophobic driving force for host-guest complexation. Isr. J. Chem. 51, 559–577 (2011)
Florea, M., Nau, W.M.: Strong binding of hydrocarbons to cucurbituril probed by fluorescent dye displacement: a supramolecular gas-sensing ensemble. Angew. Chem. Int. Ed. 50, 9338–9342 (2011)
Vinciguerra, B., Cao, L.P., Cannon, J.R., Zavalij, P.Y., Fenselau, C., Isaacs, L.: Synthesis and self-assembly processes of monofunctionalized cucurbit[7]uril. J. Am. Chem. Soc. 134, 13133–13140 (2012)
Isaacs, L., Park, S.K., Liu, S.M., Ko, Y.H., Selvapalam, N., Kim, Y., Kim, H., Zavalij, P.Y., Kim, G.H., Lee, H.S., Kim, K.: The inverted cucurbit[n]uril family. J. Am. Chem. Soc. 127, 18000–18001 (2005)
Huang, W.H., Liu, S.M., Zavalij, P.Y., Isaacs, L.: Nor-seco-cucurbit[10]uril exhibits homotropic allosterism. J. Am. Chem. Soc. 128, 14744–14745 (2006)
Ma, D., Zavalij, P.Y., Isaacs, L.: Acyclic cucurbit[n]uril congeners are high affinity hosts. J. Org. Chem. 75, 4786–4795 (2010)
Lewin, V., Rivollier, J., Coudert, S., Buisson, D.A., Baumann, D., Rousseau, B., Legrand, F.X., Kourilova, H., Berthault, P., Dognon, J.P., Heck, M.P., Huber, G.: Synthesis of cucurbit[6]uril derivatives and insights into their solubility in water. Eur. J. Org. Chem. 2013, 3857–3865 (2013)
Nally, R., Isaacs, L.: Toward supramolecular polymers incorporating double cavity cucurbituril hosts. Tetrahedron 65, 7249–7258 (2009)
Huang, W.-H., Zavalij, P.Y., Isaacs, L.: Nor-seco-cucurbit[n]uril molecular containers. Polym. Prepr. 51, 154–155 (2010)
Lemaur, V., Carroy, G., Poussigue, F., Chirot, F., De Winter, J., Isaacs, L., Dugourd, P., Cornil, J., Gerbaux, P.: Homotropic allosterism: in-depth structural analysis of the gas-phase noncovalent complexes associating a double-cavity cucurbit[n]uril-type host and size-selected protonated amino aompounds. ChemPlusChem 78, 959–969 (2013)
El-Barghouthi, M.I., Assaf, K.I., Rawashdeh, A.M.M.: Molecular dynamics of methyl viologen-cucurbit[n]uril complexes in aqueous solution. J. Chem. Theory Comput. 6, 984–992 (2010)
Rawashdeh, A.M.M., El-Barghouthi, M.I., Assaf, K.I., Al-Gharabli, S.I.: Complexation of N-methyl-4-(p-methyl benzoyl)-pyridinium methyl cation and its neutral analogue by cucurbit[7]uril and beta-cyclodextrin: a computational study. J. Incl. Phenom. Macrocycl. Chem. 64, 357–365 (2009)
Gilson, M.K.: Stress analysis at the molecular level: a forced cucurbituril-guest dissociation Pathway. J. Chem. Theory Comput. 6, 637–646 (2010)
Case, D.A., Darden, T.A., Cheatham, T.E., Simmerling, C.L., Wang, J., Duke, R.E., Luo, R., Walker, R.C., Zhang, W., Merz, K.M., Roberts, B., Wang, B., Hayik, S., Roitberg, A., Seabra, G., Kolossváry, I., Wong, K.F., Paesani, F., Vanicek, J., Liu, J., Wu, X., Brozell, S.R., Steinbrecher, T., Gohlke, H., Cai, Q., Ye, X., Wang, J., Hsieh, M.-J., Cui, G., Roe, D.R., Mathews, D.H., Seetin, M.G., Sagui, C., Babin, V., Luchko, T., Gusarov, S., Kovalenko, A., Kollman, P.A.: AMBER 11, University of California, San Francisco (2010)
Wang, J., Wolf, R.M., Caldwell, J.W., Kollman, P.A., Case, D.: Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174 (2004)
Wang, J., Wolf, R.M., Caldwell, J.W., Kollman, P.A., Case, D.: Erratum. J. Comput. Chem. 26, 114 (2005)
Jakalian, A., Bush, B.L., Jack, D.B., Bayly, C.I.: Fast, efficient generation of high-quality atomic charges. AM1-BCC model: I. Method. J. Comput. Chem. 21, 132–146 (2000)
Jorgensen, W., Chanrasekhar, J., Madura, J., Klein, M.L.: Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983)
Darden, T., York, D., Pederson, L.: Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089 (1993)
Ryckaert, J.P., Ciccotti, G., Berendsen, H.J.C.: Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 (1977)
Humphrey, W., Dalke, A., Schulten, K.: VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996)
Honig, B., Nicholls, A.: Classical electrostatics in biology and chemistry. Science 268, 1144–1149 (1995)
Jayaram, B., Sprous, D., Beveridge, D.L.: Solvation free energy of biomacromolecules: parameters for a modified generalized born model consistent with the amber force field. J. Phys. Chem. B 102, 9571–9576 (1998)
Sitkoff, D., Sharp, K.A., Honig, B.: Accurate calculation of hydration free energies using macroscopic solvent models. J. Phys. Chem. 98, 1978–1988 (1994)
Srinivasan, J., Cheatham, T.E., Cieplak, P., Kollman, P.A., Case, D.A.: Continuum solvent studies of the stability of DNA, RNA and phosphoramidate-DNA helices. J. Am. Chem. Soc. 120, 9401–9409 (1998)
Massova, I., Kollman, P.A.: Computational alanine scanning to probe protein-protein interactions: a novel approach to evaluate binding free energies. J. Am. Chem. Soc. 121, 8133–8143 (1999)
Wu, Y., Cao, Z., Yi, H., Jiang, D., Mao, X., Liu, H., Li, W.: Simulation of the interaction between ScyTx and small conductance calcium-activated potassium channel by docking and MM-PBSA. Biophys. J. 87, 105–112 (2004)
Sanner, M.F., Olson, A.J., Spehner, J.C.: Reduced surface: an efficient way to compute molecular surfaces. Biopolymers 38, 305–320 (1996)
Hou, T., Guo, S., Xu, X.: Predictions of binding of a diverse set of ligands to gelatinase-A by a combination of molecular dynamics and continuum solvent models. J. Phys. Chem. B 106, 5527–5535 (2002)
Acknowledgments
The authors wish to thank the Hashemite University for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Barghouthi, M.I., Abdel-Halim, H.M., Haj-Ibrahim, F.J. et al. Molecular dynamics of nor-Seco-cucurbit[10]uril complexes. J Incl Phenom Macrocycl Chem 82, 323–333 (2015). https://doi.org/10.1007/s10847-015-0488-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-015-0488-9